A clinical/pattern approach for barium esophagography
Images







Despite technologic advances in gastrointestinal (GI) radiology, barium esophagography remains an indispensable technique for detecting a variety of morphologic abnormalities in the esophagus, including nodules or plaques, ulcers, strictures, and rings. These abnormalities may be associated with radiographic findings that strongly suggest the underlying cause of disease. Not infrequently, however, the correct diagnosis is established only by combining the radiographic findings with the clinical history and presentation. This article therefore presents a pattern approach for esophagography based on the radiographic and clinical findings.
Technique
Double-contrast esophagography is performed as a biphasic examination that includes both double- and single-contrast views of the esophagus.1 After ingesting an effervescent agent, the patient continuously swallows high-density barium in the upright, left posterior oblique position for double-contrast views of the esophagus. A double-contrast view of the gastric cardia is also obtained in a recumbent, right side down position. The patient is then placed in a prone, right anterior oblique position and asked to take discrete swallows of low-density barium to evaluate esophageal motility. Finally, the patient continuously swallows low-density barium in the prone position to optimally distend the esophagus. The double-contrast phase of the study optimizes detection of mucosal disease, while the single-contrast phase optimizes detection of narrowing due to strictures or rings in the esophagus.
Nodules or plaques
Reflux esophagitis
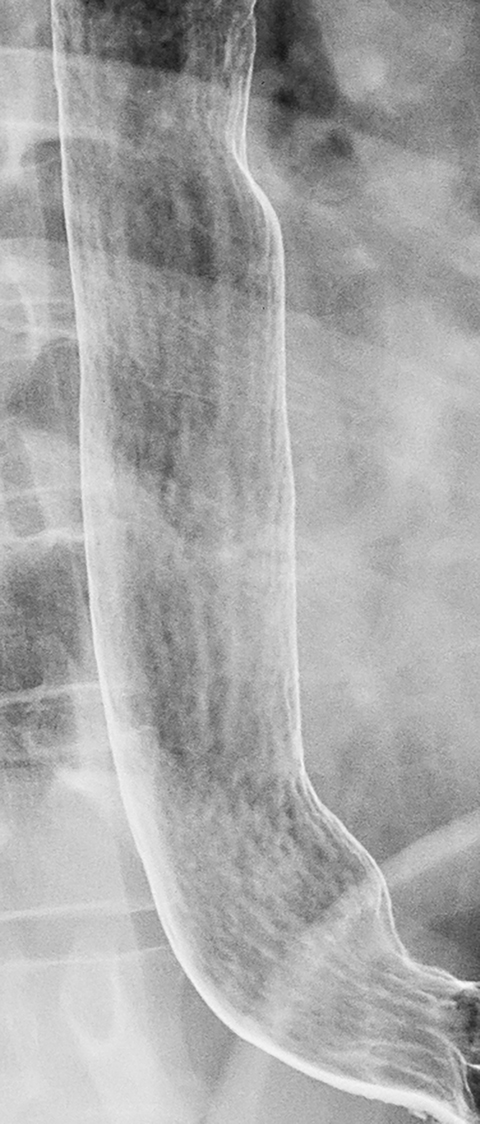
Reflux esophagitis, the most common inflammatory condition involving the esophagus, is most often manifested on double-contrast studies by a finely nodular or granular appearance caused by edema and inflammation of the mucosa. The granularity is characterized by poorly defined radiolucencies in the distal esophagus extending proximally from the gastroesophageal junction as a continuous area of disease (Figure 1).1-3 This finding is relatively sensitive and specific for reflux esophagitis, especially in patients with reflux symptoms such as heartburn, acid regurgitation, coughing and, less frequently, dysphagia or a globus sensation.3
Candida esophagitis
Candida albicans, the most common cause of infectious esophagitis, usually occurs as an opportunistic infection in patients who are immunocompromised from diabetes, malignancy, chemotherapy, AIDS or other causes.4,5 Less frequently, Candida esophagitis results from stasis caused by esophageal motility disorders such as scleroderma and achalasia that allow the fungal organism to overgrow and colonize the esophagus.6 Affected individuals typically present with acute dysphagia (difficulty swallowing) or, even more commonly, odynophagia (pain on swallowing). In some cases, the pain may be so severe that affected individuals are unable to swallow their saliva. However, only about 50% of patients have associated thrush, so the absence of oropharyngeal disease in no way excludes this diagnosis.5
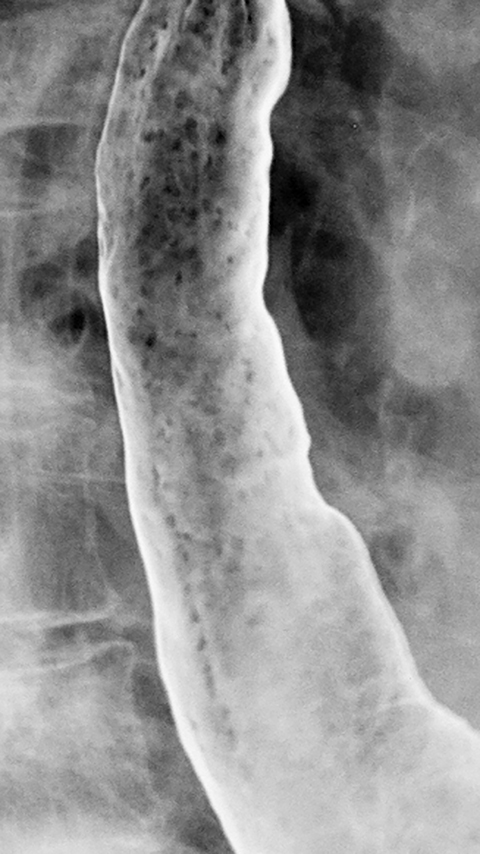
Candida esophagitis is usually manifested on double-contrast studies by multiple discrete, plaquelike defects separated by normal intervening mucosa (Figure 2A).4,5 The plaques tend to involve the upper and/or midesophagus and have a linear or irregular configuration.4,5 Patients with AIDS may develop a more fulminant form of candidiasis, with trapping of barium between innumerable plaques and pseudomembranes, producing a so-called shaggy esophagus (Figure 2B).5 This finding, which is virtually diagnostic of advanced Candida esophagitis, is not often seen in modern medical practice because of more effective therapy for HIV-positive patients.
Glycogenic acanthosis
Glycogenic acanthosis is a common degenerative condition characterized by accumulation of cytoplasmic glycogen in the squamous epithelium of the esophagus. This condition is manifested on double-contrast studies by small, rounded nodules and plaques, most often in the midesophagus (Figure 3).7 Glycogenic acanthosis may closely resemble Candida esophagitis, but the plaques of candidiasis tend to have a linear or irregular appearance, whereas the nodules of glycogenic acanthosis are more rounded. Furthermore, Candida esophagitis occurs in immunocompromised patients with odynophagia, whereas glycogenic acanthosis develops in elderly patients who are not immunocompromised and have no esophageal symptoms. Thus, it is almost always possible to differentiate these conditions on the basis of the clinical findings.
Superficial spreading carcinoma
Superficial spreading carcinoma (SSC) is an unusual form of esophageal cancer in which tumor is confined to the mucosa or submucosa, regardless of the presence or absence of lymph node metastases.8 SSC is typically manifested on double-contrast studies by a cluster of poorly defined nodules or plaques that merge with one another, producing a confluent area of disease.1,8,9 SSC can usually be differentiated from Candida esophagitis and glycogenic acanthosis, in which the plaques and nodules have discrete borders and are separated by normal intervening mucosa. When SSC is suspected on the basis of the radiographic findings, endoscopy and biopsy should be performed for a definitive diagnosis, so these patients can be treated before they develop more advanced disease.
Small ulcers
Reflux esophagitis
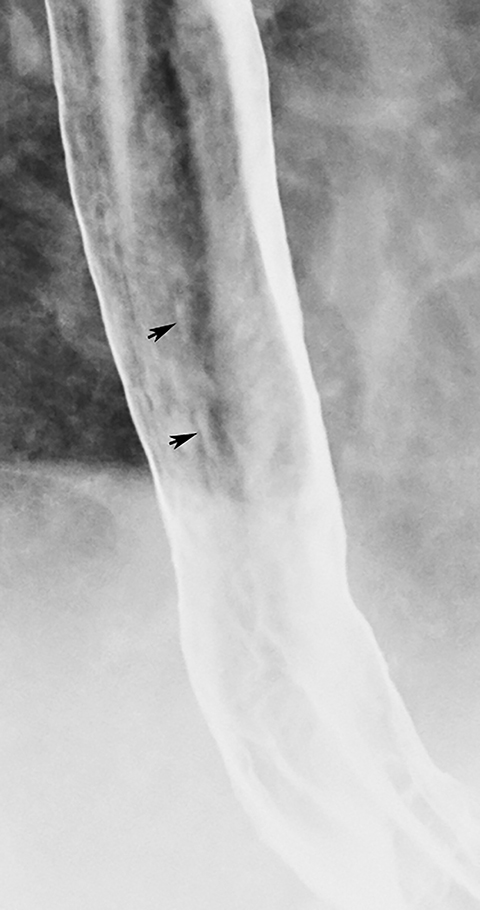
Reflux esophagitis is the most common inflammatory condition involving the esophagus. While many patients with reflux esophagitis have a finely nodular or granular mucosa (see earlier section), more advanced disease may be manifested by multiple small, shallow ulcers and erosions in the distal esophagus. The ulcers often have a punctate or linear configuration and may be associated with radiating folds or surrounding halos of edematous mucosa (Figure 4).1 The ulcers nearly always develop at or adjacent to the gastroesophageal junction, extending proximally a variable distance as a continuous area of disease.1 Ulceration that spares the distal esophagus should therefore suggest another cause of disease. Less frequently, reflux esophagitis may be manifested by a single dominant ulcer at or abutting the gastroesophageal junction. These so-called marginal ulcers are usually located on the posterior wall of the distal esophagus, most likely because of prolonged exposure to refluxed acid that pools by gravity in the posterior esophagus when the patient sleeps in a supine position.10
Herpes esophagitis
The herpes simplex virus type 1 is the second most common cause of infectious esophagitis in immunocompromised patients.5 This condition is usually manifested on double-contrast studies by multiple small ulcers in the upper or midesophagus, often surrounded by radiolucent mounds of edema (Figure 5).11,12 Most patients present with odynophagia, but herpetic lesions are not commonly found in the oropharynx, so it is difficult to differentiate herpes from Candida esophagitis on the basis of the clinical findings.
Herpes esophagitis may occasionally develop as an acute, self-limited disease in otherwise healthy patients. Affected individuals present with a flulike syndrome consisting of fever, headaches, myalgias, and upper respiratory symptoms for a period of 7-10 days prior to the sudden onset of severe odynophagia.13 Double-contrast studies typically reveal multiple tiny ulcers that are even smaller than those in immunocompromised patients with herpes esophagitis, presumably because they have an intact immune system that prevents the ulcers from enlarging.13
Drug-induced esophagitis
Patients on oral medications, particularly antibiotics such as tetracycline and doxycycline and non-steroidal anti-inflammatory drugs (NSAIDs), may develop a focal contact esophagitis. These individuals typically present with acute odynophagia and have a history of ingesting the offending medication with little or no water immediately before going to bed. As a result, the capsules or tablets may lodge in the midesophagus, where it is compressed by the aortic arch or left main bronchus. Double-contrast studies usually reveal multiple small ulcers in the midesophagus (Figure 6).1,14 This condition may be difficult to differentiate from herpes esophagitis on barium studies, but the clinical history usually suggests the correct diagnosis.
Crohn’s disease
Crohn’s disease involving the esophagus is occasionally manifested by small, superficial ulcers indistinguishable from aphthoid ulcers in the small bowel or colon in patients with this disease.15,16 Because esophageal Crohn’s disease is almost always associated with ileocolic disease, this diagnosis should only be considered in patients with known Crohn’s disease elsewhere in the GI tract.
Large ulcers
CMV esophagitis
CMV esophagitis may be manifested by one or more giant, flat ulcers that are indistinguishable from human immunodeficiency virus (HIV) ulcers in the esophagus (see next section).1 Affected individuals typically present with odynophagia and are found to have AIDS. Because of the potential toxicity of antiviral agents such as gancyclovir, endoscopic biopsies or cultures are required to confirm the presence of CMV before instituting treatment. Although better therapy for patients with the HIV virus has reduced the number of patients with AIDS, CMV esophagitis may occasionally develop in patients who receive steroids or bone marrow transplants.17-19
HIV esophagitis
Patients with HIV may develop one or more giant esophageal ulcers that are caused directly by the HIV virus itself, as confirmed on electron microscopy of biopsy specimens showing viral particles with morphologic features of HIV.20 These ulcers are usually located in the lower or midesophagus, appearing on barium studies as giant (greater than 1 cm), ovoid or diamond-shaped craters surrounded by a thin, radiolucent rim of edema (Figure 7).21,22 These ulcers are indistinguishable from giant CMV ulcers in the esophagus, but most HIV ulcers heal rapidly on treatment with steroids,21,22 whereas CMV ulcers require treatment with antiviral agents. Endoscopy and biopsy are therefore required to differentiate these infections before instituting therapy.
Drug-induced esophagitis
Unlike tetracycline- or doxycycline-induced esophagitis, which is manifested by small, shallow ulcers, esophagitis caused by potassium chloride, quinidine, NSAIDs, and alendronate sometimes leads to the development of giant esophageal ulcers.23-25 The correct diagnosis is usually suggested by the clinical history.
Barrett’s esophagus
Barrett’s esophagus is an acquired condition in which there is progressive columnar metaplasia of the distal esophagus secondary to long-standing reflux disease. Barrett’s esophagus is occasionally manifested by a single large ulcer within the columnar epithelium, occurring at a discrete distance from the gastroesophageal junction. Although uncommon, this finding should be highly suggestive of Barrett’s esophagus in a patient with a hiatal hernia and gastroesophageal reflux.1,14,26
Esophageal carcinoma
Necrotic esophageal carcinomas may be manifested by a large ulcerated mass. In such cases, barium studies may reveal a giant meniscoid or ovoid ulcer surrounded by a thick, irregular mass of tumor (Figure 8). In contrast, giant benign ulcers (e.g., CMV and HIV ulcers) have a smooth, thin rim of surrounding edema, producing a different radiographic appearance (see Figure 7).
Mimics of ulceration
Esophageal intramural pseudodiverticulosis
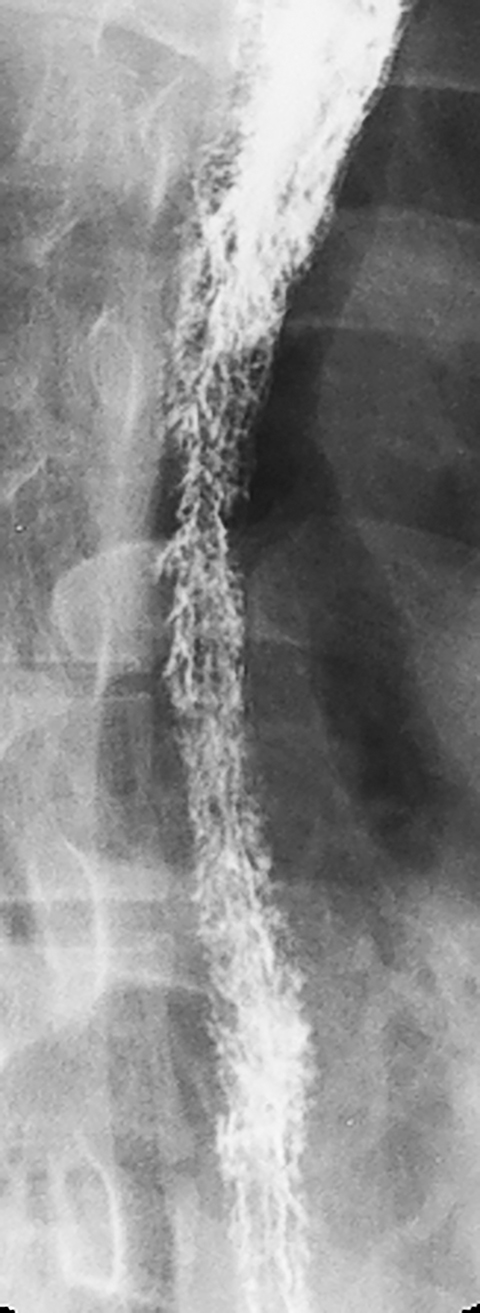
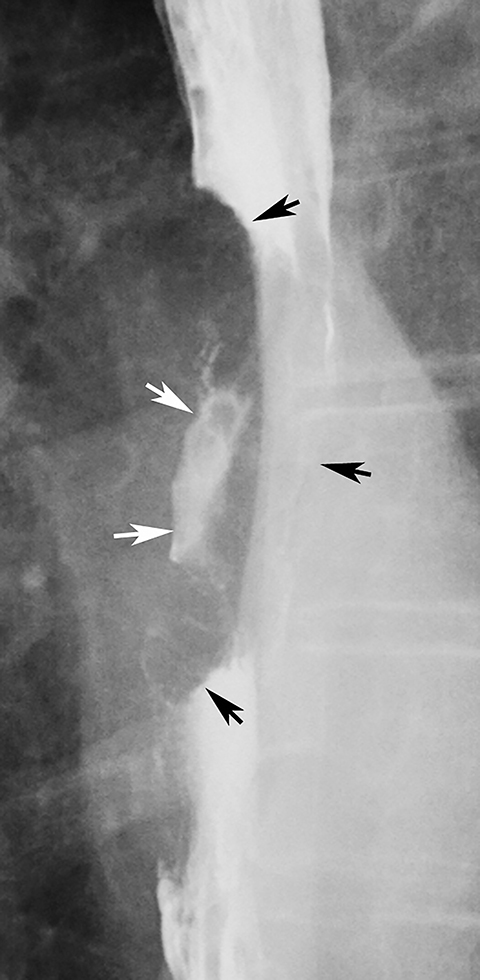
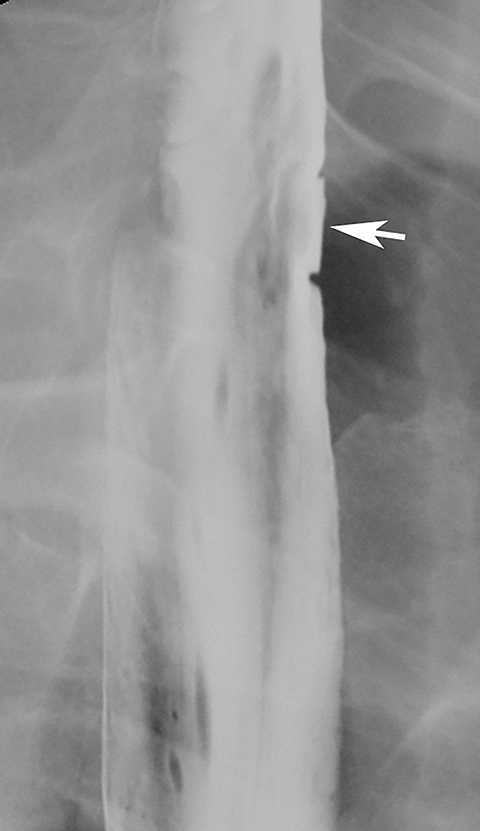
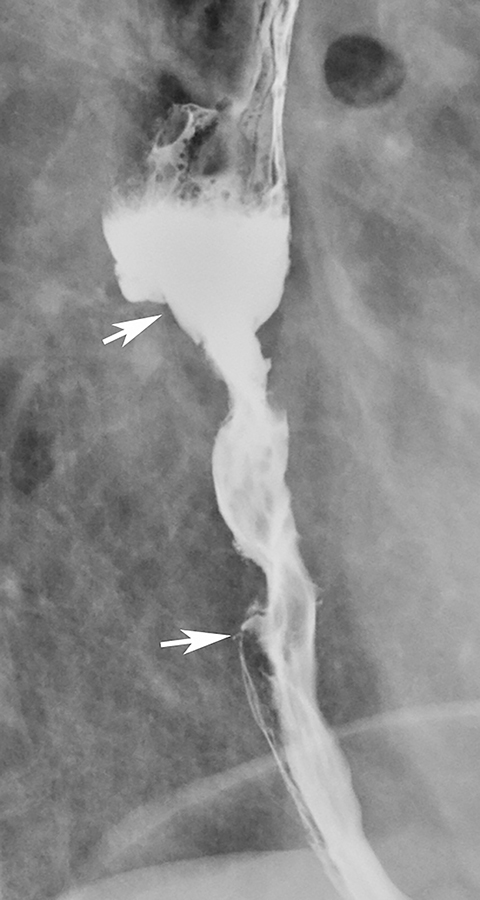
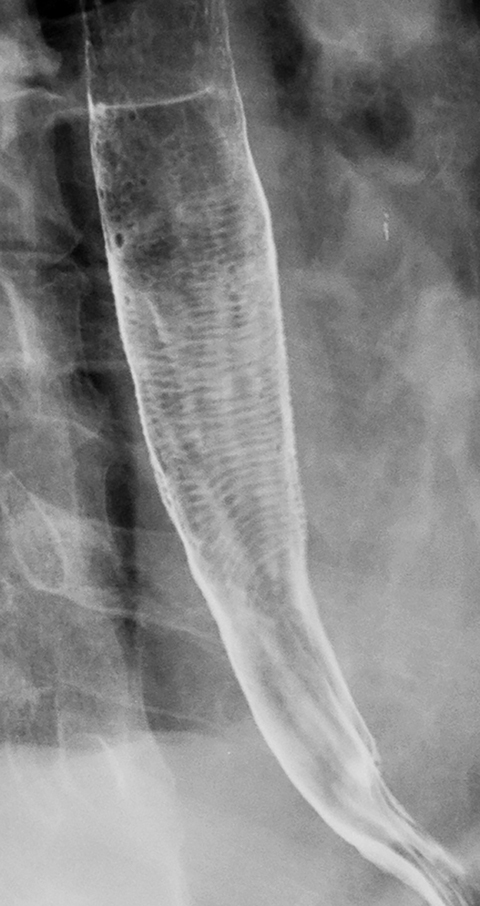
Esophageal intramural pseudodiverticula are dilated excretory ducts of deep mucous glands in the esophagus. Barium studies usually reveal multiple tiny, flask-shaped outpouchings in longitudinal rows parallel to the long axis of the esophagus (Figure 9A).27 When viewed en face, the pseudodiverticula can easily be mistaken for tiny ulcers. When viewed in profile, however, these structures often seem to be “floating” outside the esophagus, whereas true ulcers communicate directly with the lumen. Some pseudodiverticula have a diffuse distribution and are associated with high strictures (see Figure 9A), but pseudodiverticula more commonly occur in the distal esophagus in patients with peptic strictures (Figure 9B).27,28 While the pathogenesis is uncertain, it has been postulated that pseudodiverticula develop as a result of glandular dilatation from chronic inflammation. Occasionally, they may also be found in patients with alcoholism, diabetes, or Candida esophagitis.29
Ectopic gastric mucosa
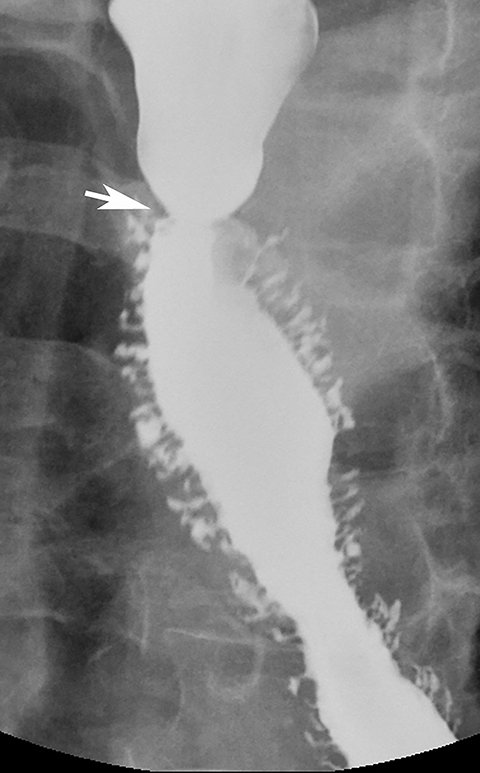
>Ectopic gastric mucosa is a common congenital anomaly unrelated to Barrett’s esophagus. It typically is discovered as an incidental finding on the right lateral or, less commonly, the left lateral wall of the upper esophagus at or near the thoracic inlet and is manifested on barium studies by a broad, flat depression, with shallow indentations at its superior and inferior margins (Figure 10).30 While this finding could be mistaken for a flat ulcer, its characteristic appearance and location should suggest the correct diagnosis. The vast majority of patients with ectopic gastric mucosa in the esophagus are asymptomatic.
Upper and midesophageal strictures
Barrett’s esophagus
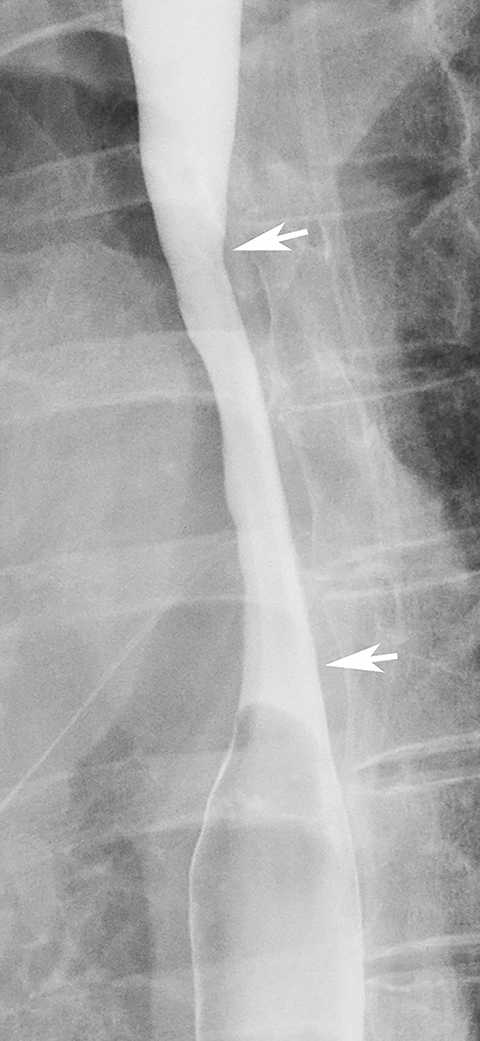
While most strictures in Barrett’s esophagus are located in the distal esophagus, some patients may develop ringlike or tapered strictures in the midesophagus (Figure 11).26 Because uncomplicated peptic strictures are almost always located within several centimeters of the gastroesophageal junction, a midesophageal stricture should be highly suggestive of Barrett’s esophagus in a patient with a hiatal hernia and reflux.26
Mediastinal irradiation
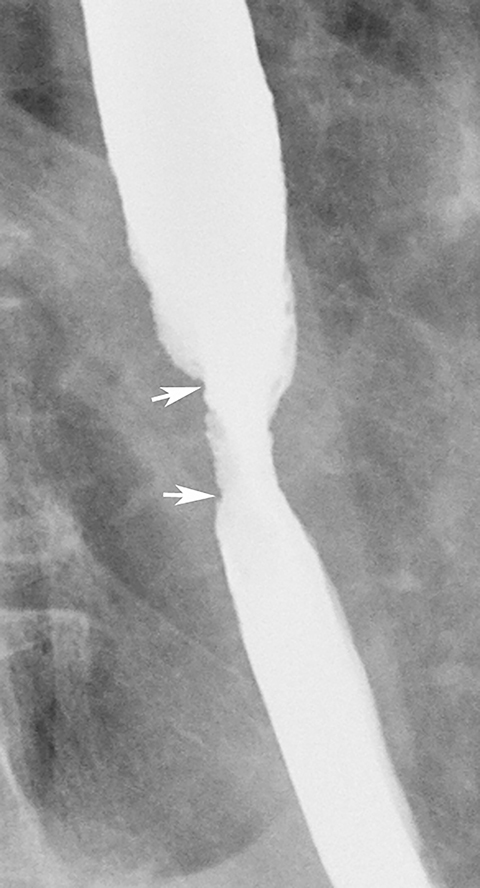
Patients who receive high doses of external beam radiation to the mediastinum may develop radiation strictures within 4 to 8 months after completion of therapy. These strictures typically appear as long segments of smooth, tapered narrowing in the upper or midesophagus within a preexisting radiation portal (Figure 12).31
Esophageal carcinoma
Malignant tumors in the upper or midesophagus are usually squamous cell carcinomas. While benign strictures typically have a smooth contour and tapered margins (Figures 11 and 12), malignant strictures have a more irregular contour and abrupt, shelflike margins, often associated with mucosal nodularity and ulceration (Figure 13).1,31 The history is also important, as patients with benign strictures have more long-standing dysphagia and little or no weight loss, whereas patients with malignant strictures have recent onset of progressive dysphagia and substantial weight loss. Thus, infiltrating carcinomas can usually be differentiated from benign strictures on the basis of the clinical and radiographic findings.
Distal esophageal strictures
Peptic stricture
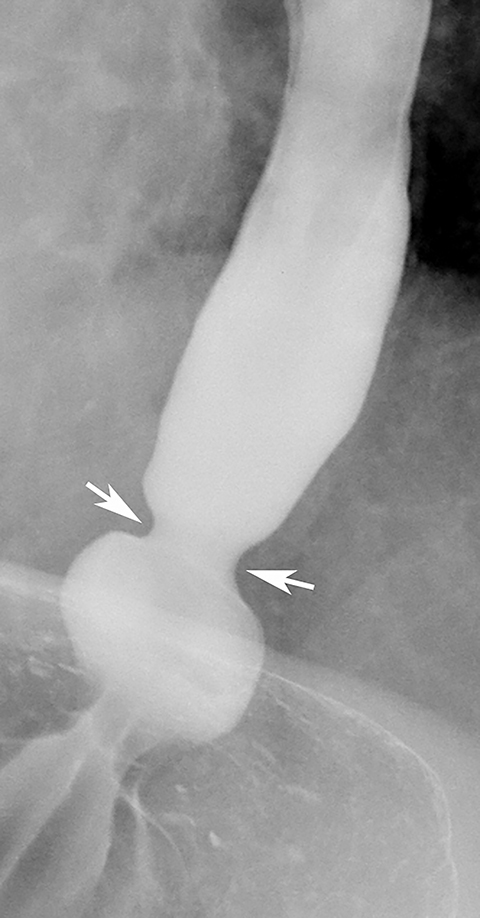
Most benign strictures in the distal esophagus are caused by scarring from reflux esophagitis.28,31 These reflux-induced or so-called peptic strictures most commonly appear as discrete (1-4 cm in length) segments of smooth, tapered narrowing, almost always above a hiatal hernia (Figure 9B). Not infrequently, however, peptic strictures are short (less than 1 cm in length), ring-like constrictions at or near the gastroesophageal junction . Such strictures can be mistaken for Schatzki rings (Figure 14),28,31 though they tend to be more asymmetric and have a slightly greater height than true rings. Conversely, nasogastric intubation, Zollinger-Ellison syndrome, and alkaline reflux esophagitis may result in rapidly progressive reflux-type strictures involving a much longer segment of the distal esophagus than most peptic strictures.28 Peptic strictures that have an unequivocally benign radiographic appearance are virtually always found to be benign, but strictures that are nodular, irregular, or asymmetric should be evaluated by endoscopy and biopsy to rule out malignant tumor.
Adenocarcinoma
Esophageal adenocarcinomas arise in areas of preexisting columnar metaplasia within Barrett’s esophagus and therefore tend to be located in the distal esophagus. Advanced adenocarcinomas are usually infiltrating lesions that narrow the lumen and, unlike squamous cell carcinomas, have a marked tendency to invade the gastric cardia and fundus.1 These lesions typically appear on barium studies as irregular areas of luminal narrowing with shelflike margins (Figure 15). Occasionally, however, an early adenocarcinoma may be recognized by nodularity or irregularity within a preexisting peptic stricture.1 Endoscopy and biopsy are required to rule out malignant tumor in these patients.
Diffuse esophageal narrowing
Eosinophilic esophagitis
Eosinophilic esophagitis is an inflammatory condition, usually occurring in children or young adults (especially men) with long-standing dysphagia and recurrent food impactions. Affected individuals often have an atopic history, asthma, and/or peripheral eosinophilia.32 Some patients have smooth, long-segment narrowing or diffuse loss of distensibility of the entire thoracic esophagus (without a discrete stricture), producing a so-called small-caliber esophagus (Figure 16A).33 Other patients have multiple distinctive ringlike indentations (sometimes associated with a focal stricture or diffuse esophageal narrowing), producing a so-called ringed esophagus (Figure 16B).34 In a young man with long-standing dysphagia and an atopic history, a small-caliber or ringed esophagus should be highly suggestive of eosinophilic esophagitis.
Caustic ingestion
Ingestion of a strong acid or base may cause severe esophagitis, leading to stricture formation within 1-3 months. Lye strictures are manifested by segmental narrowing of the upper or midesophagus or, in advanced cases, by diffuse, marked narrowing of nearly the entire thoracic esophagus (Figure 17).28,31 The small-caliber esophagus of eosinophilic esophagitis may produce similar findings (see Figure 16A), but is not usually associated with as much narrowing and irregularity as a severe lye stricture. In problematic cases, the correct diagnosis can almost always be made from the clinical history.
Esophageal rings
Feline esophagus
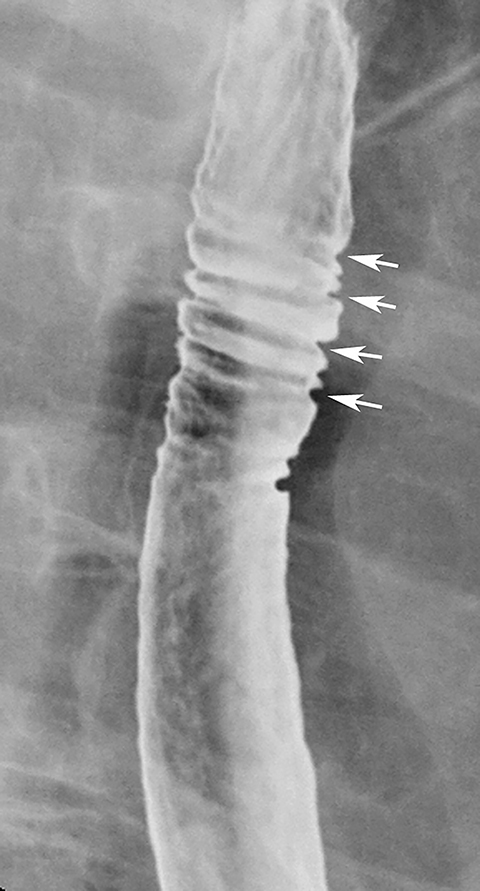
The feline esophagus is manifested on barium studies by closely spaced, thin, horizontal striations extending across the circumference of the esophagus (Figure 18).35 While the feline esophagus may be discovered as an incidental finding, it is nearly always associated with gastroesophageal reflux and is usually observed during actual reflux episodes.35 The characteristic appearance and transient nature of the feline esophagus enable differentiation from other types of esophageal rings.
Fixed transverse folds
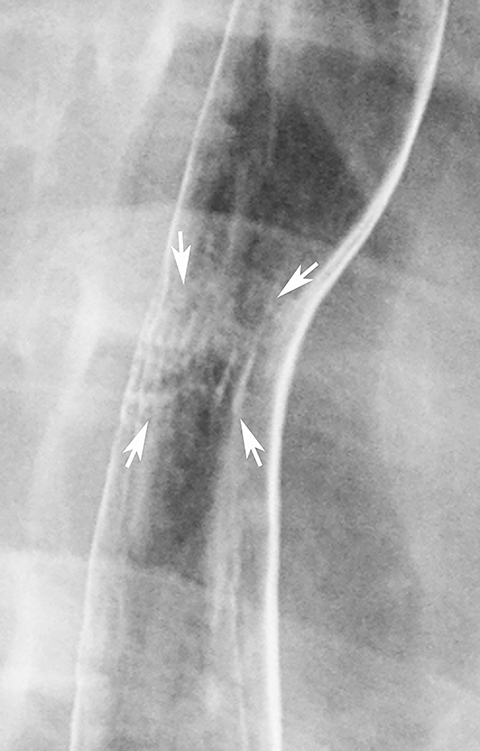
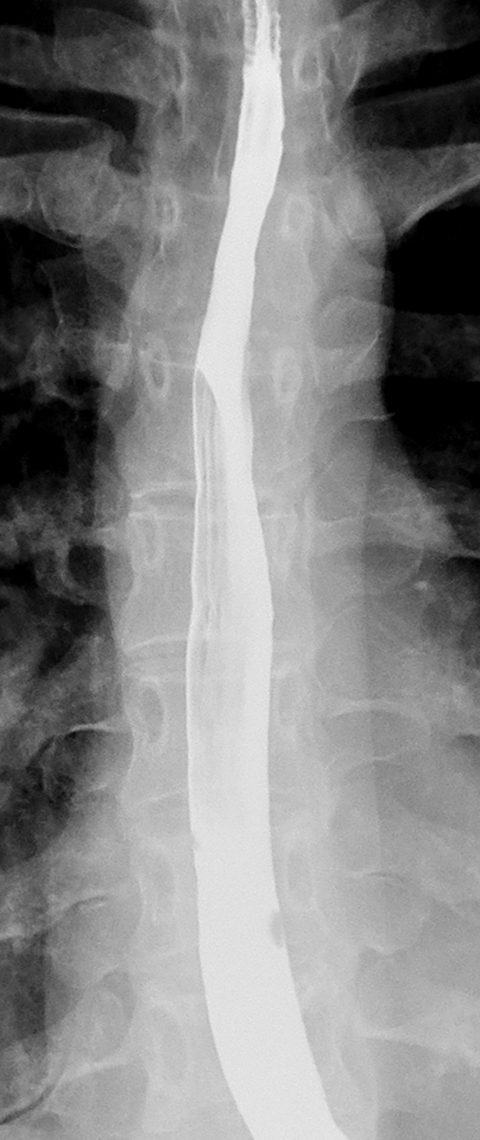
Double-contrast barium studies may occasionally reveal fixed transverse folds in the distal esophagus, with trapping of barium between the folds, producing a characteristic stepladder appearance (Figure 19).36 The folds are usually 2 to 5 mm in width and do not extend fully across the esophagus.36 They almost always develop in the region of a peptic stricture and most likely result from longitudinal scarring and shortening of the esophagus due to chronic reflux esophagitis (see Figure 19).36 Unlike the feline esophagus and nonperistaltic esophageal contractions, which are transient in nature, these transverse folds are seen as a persistent finding on esophagography.36
Ringed esophagus of eosinophilic esophagitis
As discussed earlier, patients with eosinophilic esophagitis may develop distinctive ringlike indentations (sometimes associated with a focal stricture or diffuse esophageal narrowing), producing a ringed esophagus (see Figure 16).34 While the pathogenesis of the rings is unknown, this appearance should be highly suggestive of eosinophilic esophagitis, particularly in young men with long-standing dysphagia, recurrent food impactions, and a history of allergies or asthma.
References
- Levine MS, Rubesin SE. Diseases of the esophagus: Diagnosis with esophagography. Radiology. 2005;237:414-427.
- Graziani L, Bearzi I, Romagnoli A, et al. Significance of diffuse granularity and nodularity of the esophageal mucosa at double-contrast radiography. Gastrointest Radiol. 1985;10:1-6.
- Dibble C, Levine MS, Rubesin SE, et al. Detection of reflux esophagitis on double-contrast esophagrams and endoscopy using the histologic findings as the gold standard. Abdom Imaging. 2004;29:421-425.
- Levine MS, Macones AJ, Laufer I. Candida esophagitis: Accuracy of radiographic diagnosis. Radiology. 1985;154:581-587.
- Levine MS. Infectious esophagitis. Semin Roentgenol. 1994;29:341-350.
- Gefter WB, Laufer I, Edell S, Gohel VK. Candidiasis in the obstructed esophagus. Radiology. 1981;138:25-28.
- Glick SN, Teplick SK, Goldstein J, et al. Glycogenic acanthosis of the esophagus. AJR Am J Roentgenol. 1982;139:683-688.
- Lee, SS, Ha Hk, Byun JH, et al. Superficial esophageal cancer: Esophagographic findings correlated with histopathologic findings. Radiology. 2005;236:535-544.
- Itai Y, Kogure T, Okuyama Y, Akiyama H. Diffuse finely nodular lesions of the esophagus. AJR Am J Roentgenol. 1977;128:563-566.
- Hu C, Levine MS, Laufer I. Solitary ulcers in reflux esophagitis: Radiographic findings. Abdom Imaging. 1997;22:5-7.
- Levine MS, Laufer I, Kressel HM, Friedman HM. Herpes esophagitis. AJR Am J Roentgenol. 1981;136:863-866.
- Levine MS, Loevner LA, Saul SH, et al. Herpes esophagitis: Sensitivity of double-contrast esophagography. AJR Am J Roentgenol. 1988;151:57-62.
- Shortsleeve MJ, Levine MS. Herpes esophagitis in otherwise healthy patients: Clinical and radiographic findings. Radiology. 1992;182:859-861.
- Levine MS, Rubesin SE, Laufer I. Barium esophagography: A study for all seasons. Clin Gastroenterol Hepatol. 2008;6:11-25.
- Gohel V, Long BW, Richter G. Aphthous ulcers in the esophagus with Crohn colitis. AJR Am J Roentgenol. 1981;137:872-873.
- Degryse HR, De Schepper AM. Aphthoid esophageal ulcers in Crohn’s disease of ileum and colon. Gastrointest Radiol. 1984;9:197-201.
- Akin S, Tufan F, Bahat G, et al. Cytomegalovirus esophagitis precipitated with immunosuppression in elderly giant cell arteritis patients. Aging Clin Exp Res. 2013;25:215-218.
- Moosig F, Gross WL. Esophagitis during immunosuppression. Z Rheumatol. 2012;71:326-327.
- Fiegl M, Gerbitz A, Gaeta A, et al. Recovery from CMV esophagitis after allogeneic bone marrow transplantation using non-myeloablative conditioning: The role of immunosuppression. J Clin Virol. 2005;34:219-223.
- Rabeneck L, Popovic M, Gartner S, et al. Acute HIV infection presenting with painful swallowing and esophageal ulcers. JAMA. 1990;263:2318-2322.
- 21. Sor S, Levine MS, Kowalski TE, et al. Giant ulcers of the esophagus in patients with human immunodeficiency virus: Clinical, radiographic, and pathologic findings. Radiology. 1995;194:447-451.
- Levine MS, Loercher G, Katzka DA, et al. Giant, human immunodeficiency virus-related ulcers in the esophagus. Radiology. 1991;180:323-326.
- Zografos GN, Georgiadou D, Thomas D, et al. Drug-induced esophagitis. Dis Esophagus. 2009;22:633-637.
- Levine MS, Rothstein RD, Laufer I. Giant esophageal ulcer due to Clinoril. AJR Am J Roentgenol. 1991;156:955-956.
- Ryan JM, Kelsey P, Ryan BM, Mueller PR. Alendronate-induced esophagitis: Case report of a recently recognized form of severe esophagitis with esophageal stricture--radiographic features. Radiology. 1998;206:389-391.
- Levine MS. Barrett esophagus: update for radiologists. Abdom Imaging. 2005;30:133-141.
- Levine MS, Moolten DN, Herlinger H, Laufer I. Esophageal intramural pseudodiverticulosis: A reevaluation. AJR Am J Roentgenol. 1986;147:1165-1170.
- Luedtke P, Levine MS, Rubesin SE, et al. Radiologic diagnosis of benign esophageal strictures: A pattern approach. RadioGraphics. 2003;23:897-909.
- Cho SR, Sanders MM, Turner MA, et al. Esophageal intramural pseudodiverticulosis. Gastrointest Radiol. 1981;6:9-16.
- Takeji H, Ueno J, Nishitani H. Ectopic gastric mucosa in the upper esophagus: Prevalence and radiologic findings. AJR Am J Roentgenol. 1995;164:901-904.
- Gupta S, Levine MS, Rubesin SE, et al. Usefulness of barium studies for differentiating benign and malignant strictures of the esophagus. AJR Am J Roentgenol. 2003;180:737-744.
- Vasilopoulos S, Murphy P, Auerbach A, et al. The small-caliber esophagus: An unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc. 2002;55:99-106.
- White SB, Levine MS, Rubesin SE, et al. The small-caliber esophagus: Radiographic sign of idiopathic eosinophilic esophagitis. Radiology. 2010;256:127-134.
- Zimmerman SL, Levine MS, Rubesin SE, et al. Idiopathic eosinophilic esophagitis in adults: The ringed esophagus. Radiology. 2005;236: 159-165.
- Samadi F, Levine MS, Rubesin SE, et al. Feline esophagus and gastroesophageal reflux. AJR Am J Roentgenol. 2010;194:972-976.
- Levine MS, Goldstein HM. Fixed transverse folds in the esophagus: A sign of reflux esophagitis. AJR Am J Roentgenol. 1984;143:275-278.