A problem-solving approach to common challenges interpreting postoperative spinal imaging
By Stechishin ODM, Vertinsky AT, Street J, Shewchuk JR
Spinal surgery is a relatively common medical procedure with approximately 1.2 million operations occurring annually in the U.S.1 Imaging plays a critical role in routine surveillance and assessment of new and recurrent symptoms after spinal surgery. Consequently, the postoperative spine is commonly encountered in specialized and general radiology practices.
Interpretation of postoperative imaging is complex given the patient’s underlying preoperative spinal pathology, the altered postsurgical anatomy, and the artifact caused by hardware. In this review, we focus on common, clinically challenging scenarios in the interpretation of postoperative spinal imaging, including evaluating hardware complications, assessing for segmental fusion, detecting spinal infection, differentiating paraspinal fluid collections, and separating recurrent disc disease from peridural fibrosis.
Hardware complication or acceptable postsurgical appearance?
Evaluation of spinal hardware integrity is the most common indication for postoperative surveillance imaging. Hardware-related complications, including implant fracture, loosening, migration and subsidence, are relatively common, estimated to occur in 2 to 40% of spinal fusion patients.2 Persistent motion, pseudoarthrosis, and infection are common predisposing factors to hardware failure. While the diagnosis is relatively straightforward when there is frank hardware failure, subtler alterations can pose a dilemma as to whether findings are within the range of acceptable postoperative appearances.
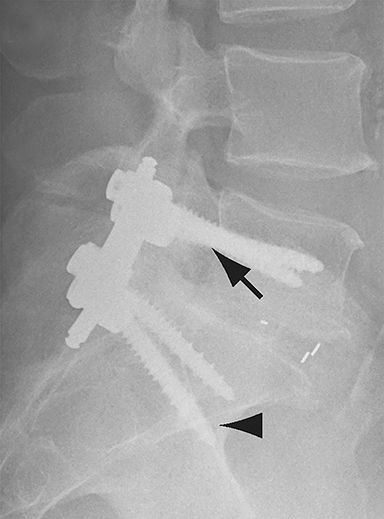
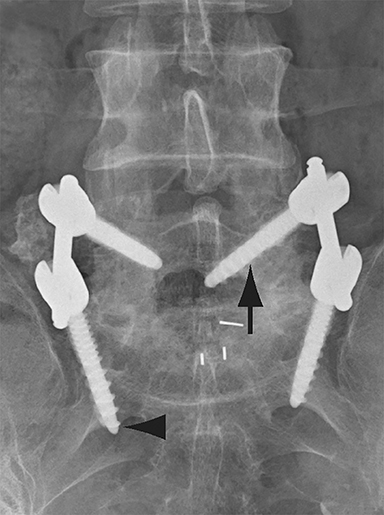
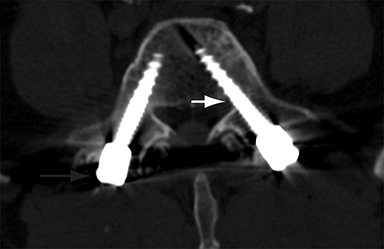
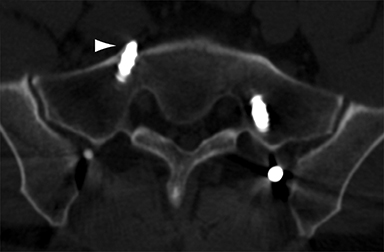
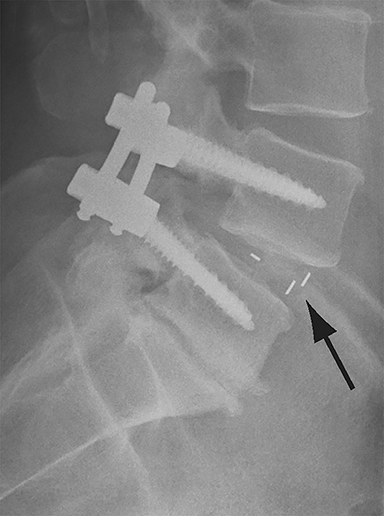
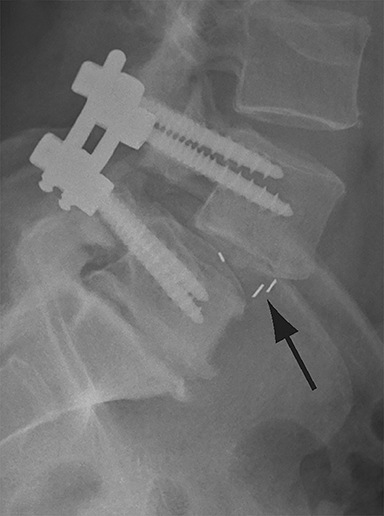
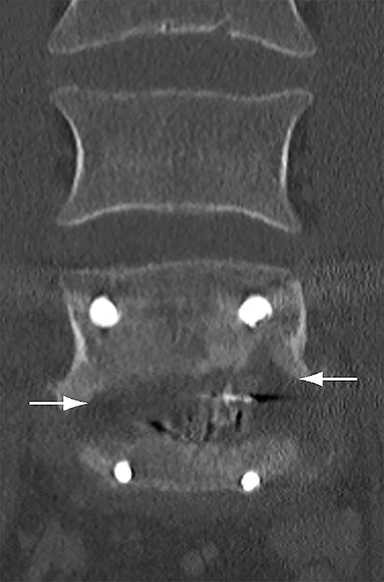
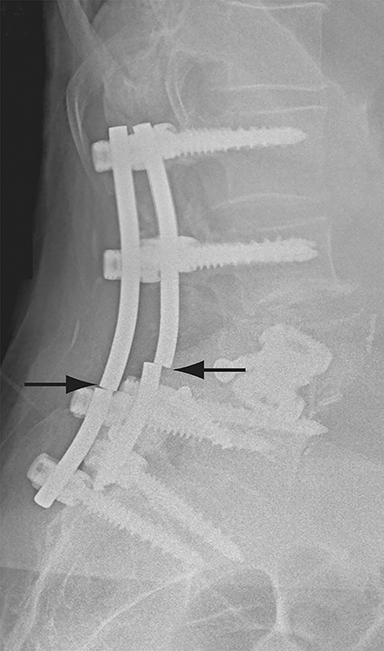
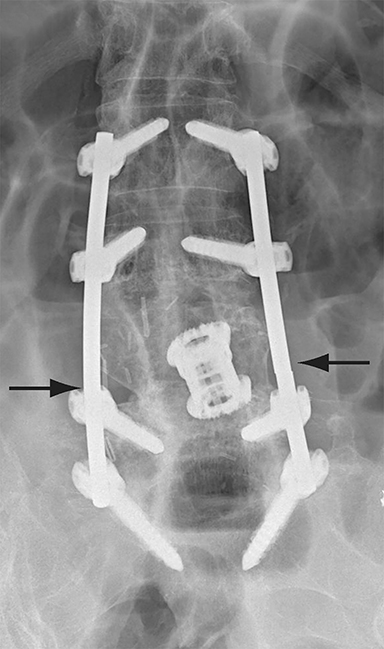
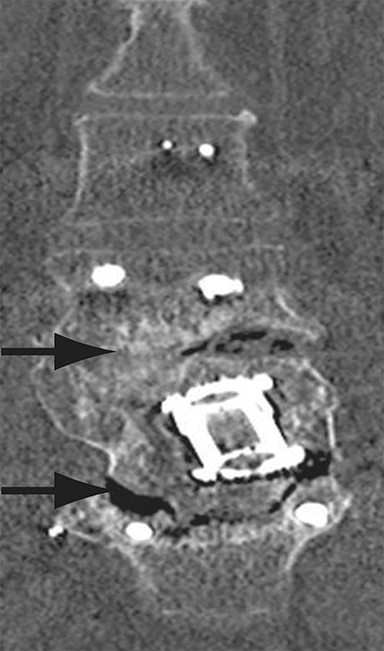
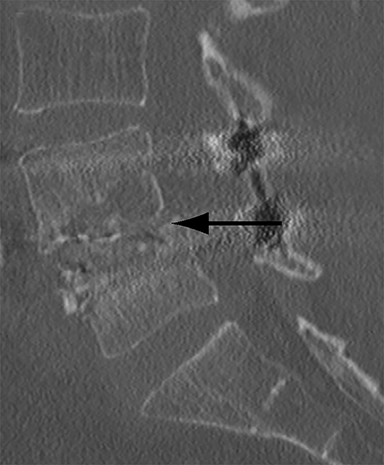
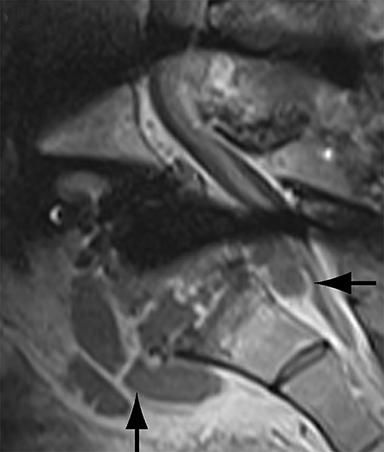
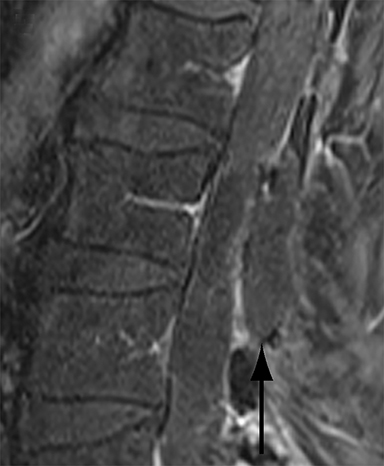
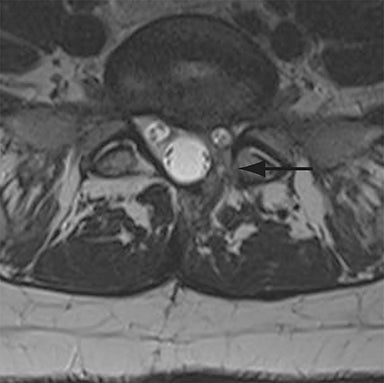
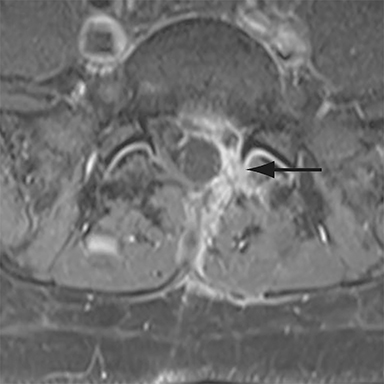
Malpositioned screws constitute the most common hardware-related complication. In anterior or lateral fixation, the screw tips should lie within the vertebral body. Traversing the contralateral cortex could violate the spinal canal or adjacent major vessels or organs, while penetration of the endplate would violate the disc.3,4 Pedicle screws used in posterior fixation should ideally traverse the pedicle along a course either parallel to the endplate (straight forward trajectory) or parallel to the pedicle long axis (anatomical trajectory), with their tips remaining within the vertebral body. Screw tips that protrude through the anterior cortex of the vertebral body risk injury to adjacent major vessels or organs. Similarly, pedicle screws that violate the medial or inferior cortices may injure or irritate the spinal cord or a nerve root descending in the subarticular recess or exiting from the neural foramen (Figure 1).5
Frank hardware fracture, usually involving fracture of screws or posterior rods, is relatively rare. Pedicle screw fracture is estimated to occur in 0.5% of cases.6 Iliac screw fracture is more common, occurring in 8-22% of cases, and usually signifies failure of fusion at the lumbosacral junction.7 Rod fracture is seen in 9-16% of postoperative cases of major adult spinal deformity.8 Screw loosening is suspected when a radiolucent rim is ≥2 mm in width or progressively enlarges over time. In the late postoperative period, hardware migration can result from bone remodeling, chronic hardware-related pressure erosions or failure of segmental fusion. Screws initially well-positioned may end up protruding through the endplate into the disc, or through the pedicle or vertebral body resulting in direct damage to nearby neurovascular structures or development of pressure-related bursae that may impinge adjacent nerves or vessels.
Complications related to interbody cage disc spacer placement involve cage migration, displacement, subsidence, and endplate fracture (Figure 1). Interbody disc cage spacers should lie anterior to the posterior vertebral body margin by at least 1/4 of the endplate length to minimize the potential for spinal canal narrowing and thecal sac impingement. Subsidence is defined as more than 3 mm of migration of the interbody disc spacer into the vertebral endplate, resulting in loss of disc space height.9 Morselized bone autograft will not be visible on plain radiographs; thus, the appearance of an interbody implant floating in the disc space does not necessarily indicate bone-graft failure in the early postoperative period.
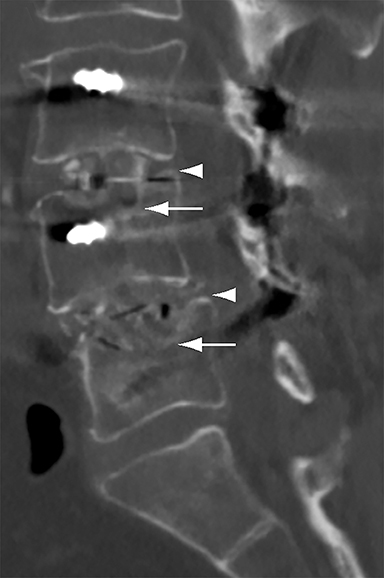
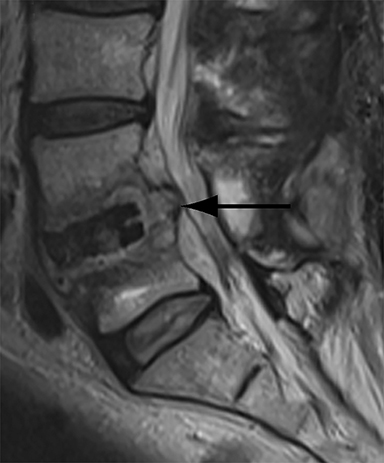
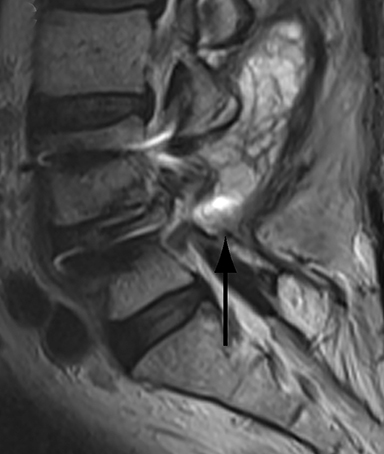
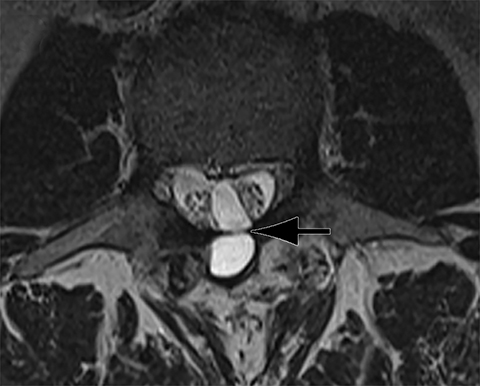
Patients undergoing recombinant human bone morphogenetic protein (rhBMP) assisted spinal fusion merit a special discussion, as the expected postoperative appearance differs significantly from that of traditional fusion procedures. Endplate osteolysis and vertebral body bony defects are common in these patients, occurring in 100% of cervical spine fusion and 82% of lumbar spine fusions at 6 weeks in a recent case series (Figure 2).10 The inflammatory reaction incited by rhBMP is dose related and also induces increased prevertebral soft-tissue swelling within the first 6 weeks as compared to traditional fusion procedures.10 This aggressive inflammatory reaction, together with nearly universal endplate osteolysis, can easily be mistaken for either sterile spondylodiscitis or deep surgical site infection.11 Endplate osteolysis and rapid bone turnover also frequently result in movement of the interbody cage spacer with subsidence or migration, occurring in nearly half of patients between 1.5 and 3 months after surgery.10 Additionally, exuberant new bone formation induced by rhBMP may extend into the neural foramen resulting in new foraminal stenosis and radiculopathy on the side from which the interbody disc spacer containing the rhBMP was inserted.12
Failed fusion or too early to see radiographic fusion?
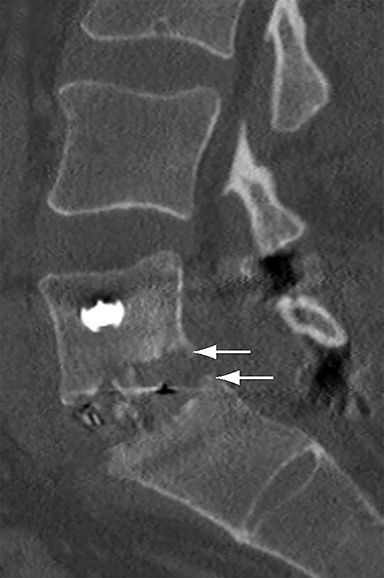
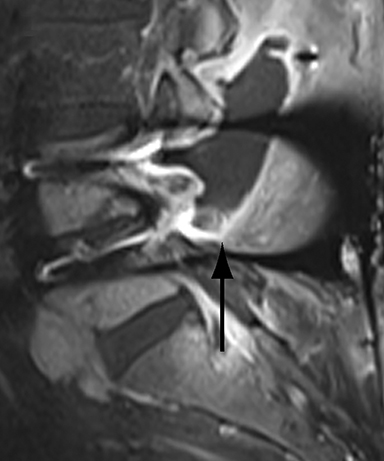
Assessing the extent of fusion, or lack thereof, is a common imaging indication in the late postoperative period. Greater than 3 of motion between flexion and extension views obtained 8-16 weeks after surgery is suggestive of failed fusion.9 Radiographically, osseous fusion is defined by the development of bridging trabecular bone. The ‘time to fusion’ varies with age and with anatomical site and morphometric location. In adults, posterolateral or facet fusions take longer than interbody fusions (9-12 months versus 3-9 months) with fusions in the cervical spine occurring faster than thoracic or lumbar spine.13 Premineralized osteoid may allow a functional fusion, but is radiolucent; thus, functional fusion on flexion-extension views may precede osseous fusion by several months.
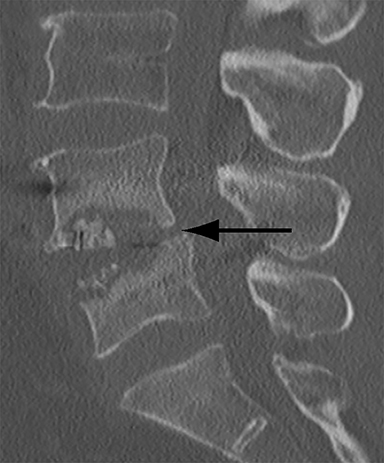
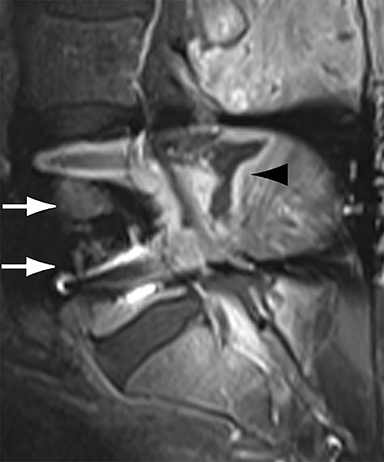
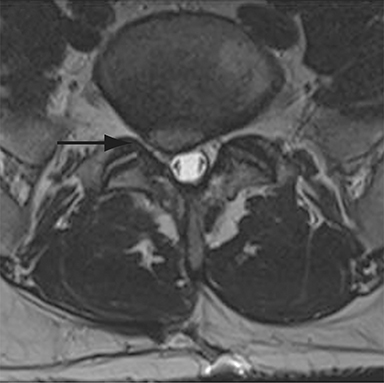
With interbody fusion, trabecular bridging begins to develop initially outside of the interbody cage spacer by 3 months and should completely bridge the intervertebral disc space by 6 months after cervical and 9 months after lumbar surgery.14 Radiographic evidence of fusion will appear sooner in patients undergoing rhBMP-assisted procedures, and definitely should be evident by 6 months.10 Central trabecular disruption or misalignment suggests delayed union with ongoing motion, which can lead to development of a pseudoarthrosis (Figure 3). On MRI, persistent low T1 and high T2 signal between the vertebral body and bone graft beyond 6 months after surgery is suggestive of fusion failure and early pseudoarthrosis formation. Increased radiotracer uptake on bone scan beyond 12 months after surgery further corroborates the presence of a pseudoarthrosis.14 On CT, the absence of mature trabeculations crossing the disc space at 24 months is diagnostic of failed fusion.15
For posterolateral fusion, the initial appearance on CT or MR will be of numerous morselized bone fragments packed between the transverse processes and adjacent to the facet joints. Over subsequent months, there will be progressive bony bridging between the fragments and the transverse processes and facets. The lack of complete osseous coalition or presence of a persistent discontinuity in the bony bridge after 24 months is diagnostic of failed or incomplete fusion.16,17
Infection or postoperative change?
The clinical diagnosis of surgical site infection (SSI) can often be challenging. There may or may not be wound discharge, although this is more typical in SSI after instrumentation. Surgical site infection typically presents between postoperative days 4 and 28. Temperature is unreliable as pyrexia is often seen with post-anesthetic metabolic derangement, atelectasis or any remote nonsurgical site infection. Rising CRP beyond 96 hours is characteristic.18 The diagnosis is usually made using a combination of clinical, laboratory and radiographic features.
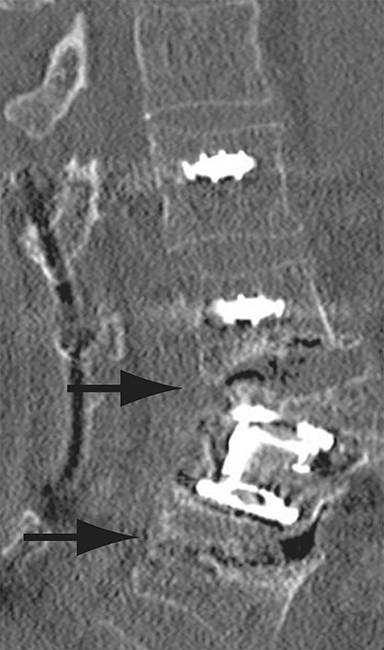
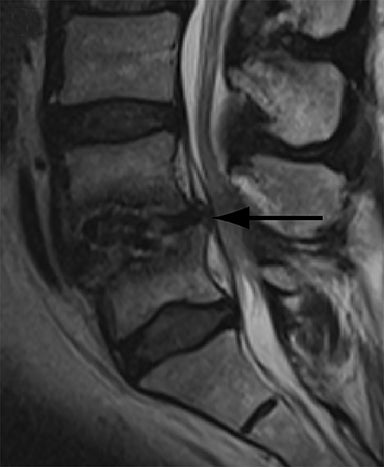
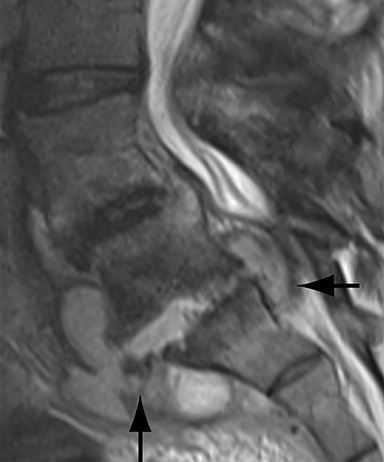
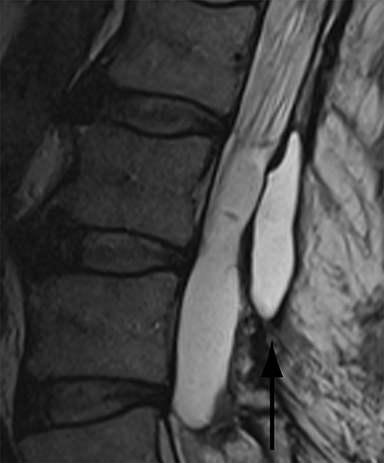
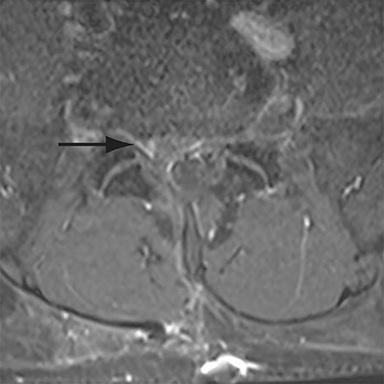
Thus, although postoperative spondylodiscitis is relatively uncommon, with an estimated incidence of 0.2 – 2.8%, excluding infection is a relatively common indication for both surveillance imaging and investigation of new postoperative symptoms.19 While radiographs are often the initial imaging investigation, excluding spondylodiscitis or vertebral osteomyelitis is essentially impossible given the low sensitivity of radiographs for early infection in the spine.5 Subtle loss of subchondral endplate definition and endplate erosions are the most reliable initial radiographic findings, but are rarely present in the first 2 weeks.20 Progressive infiltration of the vertebral marrow with infection will result in osteopenia, although this is not evident radiographically until there has been at least 60% demineralization.20 Late findings of spondylodiscitis occurring after 6 weeks include frank bony destruction with loss of intervertebral disc space leading to eventual endplate sclerosis and fusion across the disc space.21 CT has much greater sensitivity than radiographs for detecting early endplate erosions as well as adjacent paraspinal inflammatory stranding or abscess formation (Figure 4).20,22
Magnetic resonance imaging is the most sensitive modality to identify early findings of postoperative spondylodiscitis. T1-weighted images demonstrate subchondral endplate irregularity and erosions. Adjacent bone marrow edema and purulent infiltration typically appear as low T1 signal and high T2 signal with enhancement following intravenous gadolinium administration. Infected intervertebral discs demonstrate low T1 signal and high T2 signal with diffuse homogeneous, patchy, or peripheral contrast enhancement.23,24 Surrounding mixed T1 and T2 signal paravertebral inflammatory change is frequently present.23
The findings of spondylodiscitis need to be interpreted with caution as postoperative or degenerative change can mimic spondylodiscitis. In rhBMP-assisted fusions, endplate osteolysis and exuberant paravertebral soft-tissue swelling are frequent and expected changes that can be easily misinterpreted as infection (Figure 4).10 Similarly, high T2 signal with variable enhancement in an intervertebral disc can be a normal finding for up to 6 months post-discectomy in the absence of infection.25 Linear disc enhancement is more likely to be postsurgical whereas peripheral enhancement of the residual disc with reactive endplate changes points to infection.26,27 Similarly, nerve root enhancement at 3-6 weeks after surgery is a relatively frequent finding, occurring in 20-62% of patients, but persistence of enhancement beyond 6 weeks is abnormal.28 Junctional failure due to accelerated degenerative change at the levels adjacent to the fused segments can mimic spondylodiscitis due to vertebral body marrow edema, which may enhance.23 The presence of enhancing paravertebral or epidural soft tissue greatly increases the likelihood of septic spondylodiscitis.29,30 Restricted diffusion within the disc space and endplates is also consistent with spondylodiscitis, but not degenerative change.31
Nuclear medicine scans can be helpful in equivocal cases of possible infection. Bone scan with technetium-99m-diphosphonates is relatively sensitive for spondylodiscitis, classically demonstrating increased activity on blood pool and delayed static images centered at a disc space.32 However, bone scan has poor specificity with a similar pattern present in degenerative or inflammatory spondyloarthropathy.32 Gallium-67-citrate scintigraphy demonstrates greater specificity for infection and the combination of bone scan with Ga-67 SPECT/CT has been reported to achieve sensitivity and specificity for spondylodiscitis comparable to MRI.33 However, the specificity of this technique in the early postoperative period is compromised by uptake at the surgical incision site during the first two weeks postoperatively in the absence of infection.34 Labeled white blood cell scans are of limited utility with poor sensitivity for spondylodiscitis due to significant normal uptake of leukocytes in noninfected vertebral marrow and poor uptake in encapsulated paraspinal infection.35 In the last few years, 18FFDG PET/CT has emerged as the nuclear medicine technique of choice offering good sensitivity and specificity for spondylodiscitis comparable to MRI.36 18FFDG PET/CT may be particularly useful in ruling out spondylodiscitis in cases equivocal on MRI as a recent prospective study reported a sensitivity of 100% for identifying spondylodiscitis with the two techniques together.37
Abscess or sterile postoperative fluid collection?
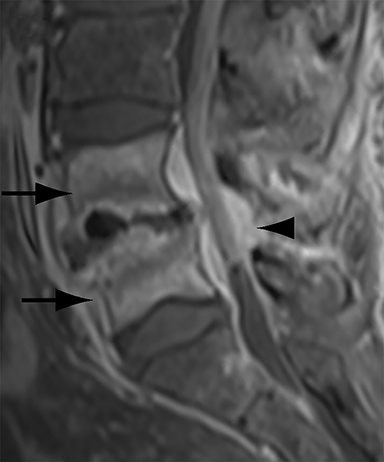
Differentiating abscess from non-infected postoperative hematoma, seroma or pseudomeningocele has significant clinical implications. With progression of infection, heterogeneously enhancing T1 low signal and T2 high signal paraspinal soft tissue organizes into a peripherally enhancing T1 low/intermediate signal and T2 high signal fluid collection (Figure 5). Adjacent features of spondylodiscitis (as described in the preceding section) are usually present as most epidural abscesses result from direct extension of spondylodiscitis. Similarly, a psoas or paraspinal abscess can develop as a complication of spondylodiscitis.
Confident identification of superimposed infection in a postoperative hematoma or seroma can be challenging at times. Thin peripheral contrast enhancement may be a normal finding in non-infected hematomas and seromas, but irregularly enhancing walls or adjacent inflammatory change strongly points to infection.1 Diffusion weighted imaging can help to identify infected paraspinal collections with developing abscesses demonstrating restricted diffusion.38 However, it should be noted that chronic hematomas may also demonstrate restricted diffusion due to viscous blood breakdown products.38 Unrepaired postsurgical dural leaks resulting in pseudomeningocele formation are relatively uncommon, occurring in less than 2% of all spinal surgery cases.12 Large pseudomeningoceles that extend to the skin surface are at particularly high risk for infection and cutaneous fistula formation. The key finding on MRI to distinguish pseudomeningoceles from other extra-dural fluid collections is the identification of either direct communication or flow artifact between the thecal sac and the collection (Figure 5). Thin peripheral contrast enhancement can be a normal finding, but irregular enhancement, especially with adjacent inflammatory change, is highly suspicious for infection. Ancillary findings of meningitis including leptomeningeal enhancement and intracranial increased T2 FLAIR signal in the sulci may be present with an infected pseudomeningocele. Importantly, pachymeningeal enhancement due to intracranial hypotension should not be confused with leptomeningeal enhancement indicative of meningitis.
Recurrent disc herniation or peridural fibrosis?
In postoperative discectomy patients with recurrent radicular symptoms, differentiating recurrent disc herniation from peridural fibrosis is important as reoperation will only potentially benefit the former and is relatively contraindicated in the latter case. Imaging too early in the immediate postoperative period is a potential pitfall as annulus fibrosis, epidural space edema, and granulation tissue can mimic a recurrent disc herniation.39 All patients should be evaluated with contrast enhanced MRI since both will appear as heterogeneous soft tissue effacing the epidural fat. A disc herniation demonstrates early peripheral enhancement due to granulation tissue or dilated epidural venous plexus, with central non-enhancement (Figure 6). Conversely, peridural fibrosis enhances rapidly and diffusely. It is important that the enhancement pattern be evaluated within the first 5 minutes after administration of gadolinium as disc herniation may also demonstrate diffuse enhancement on delayed post-gadolinium images.40 The presence of intermediate signal with irregular margins is more in keeping with peridural fibrosis whereas low signal with smooth margins favors recurrent herniation. In patients who may have both peridural fibrosis and recurrent disc protrusion, it can be difficult to determine the relative contributions of each to the patient’s symptoms. Peridural fibrosis is often asymptomatic, although diffuse peridural fibrosis is more likely to cause symptoms than small focal areas of peridural scarring.41,42 Of note, peridural scarring may not be apparent on MR, and in highly symptomatic patients with negative MR findings, epiduroscopy may be required to completely exclude peridural fibrosis as a cause of persistent pain.43
Conclusion
Postoperative spinal imaging is a common and potentially challenging radiological examination to accurately interpret. Postsurgical changes in the spine can simulate significant postoperative pathology. Several key imaging features are helpful to accurately assess hardware integrity, segmental fusion, infection, paraspinal fluid collections, and recurrent disc disease. Malpositioned pedicle screws that violate the inferomedial cortices of the pedicle or protrude through the anterior cortex of the vertebral body are the most common hardware complication. Endplate osteolysis and interbody spacer subsidence are expected in rhBMP assisted fusion. When assessing fusion on CT, bridging trabeculae may not be present before 6 months, but must be present by 24 months if fusion has successfully occurred.
Endplate osteolysis with progressive or persistent enhancement beyond 6 weeks is consistent with spondylodiscitis. Thin wall enhancement may be present in sterile paraspinal fluid collections, but thick enhancing walls and restricted diffusion, especially when associated with features of spondylodiscitis, are concerning for abscess. Recurrent disc herniation enhances only peripherally on images obtained within 5 minutes of gadolinium administration, while peridural fibrosis enhances diffusely. These key imaging features can help the radiologist to accurately navigate these challenging scenarios in postoperative spinal imaging and confidently distinguish postoperative changes from true pathology.
References
- Jain NK, Dao K, Ortiz AO. Radiologic evaluation and management of postoperative spine paraspinal fluid collections. Neuroimaging Clin N Am. 2014;24(2):375-389.
- Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144-157.
- Venu V, Vertinsky AT, Malfair D, et al. Plain radiograph assessment of spinal hardware. Semin Musculoskelet Radiol. 2011;15(2):151-162.
- Yeo JM, Vertinsky AT, Chew JB, et al. Imaging in adult scoliosis: preoperative assessment and postoperative complications. Semin Musculoskelet Radiol. 2011;15(2):143-150.
- Young PM, Berquist TH, Bancroft LW, et al. Complications of spinal instrumentation. Radiographics. 2007;27(3):775–789.
- Lonstein JE, Denis F, Perra JH, et al. Complications associated with pedicle screws. J Bone Joint Surg (Am Vol). 1999;81(11):1519–1528.
- Guler UO, Cetin E, Yaman O, et al. Sacropelvic fixation in adult spinal deformity (ASD), a very high rate of mechanical failure. Eur Spine J. 2015;24(5):1085-1091.
- Barton C, Noshchenko A, Patel V, et al. Risk factors for rod fracture after posterior correction of adult spinal deformity with osteotomy: a retrospective case-series. Scoliosis.2015;10:30.
- Malhotra A, Kalra VB, Wu X, et al. Imaging of lumbar spinal surgery complications. Insights Imaging. 2015;6(6):579-590.
- Sethi A, Craig J, Bartol S, et al. Radiographic and CT evaluation of recombinant human bone morphogenetic protein-2-assisted spinal interbody fusion. AJR Am J Roentgenol. 2011;197(1):W128-W133.
- Saigal G, Quencer R, Guest JD, et al. Vertebral body osteolysis following the use of bone morphogenetic protein in spinal surgery: a mimicker of infection. J Neuroradiol. 2012;39(5):354-359.
- Willson MC, Ross J. Postoperative spine complications. Neuroimaging Clin N Am. 2014;24(2):305–326.
- Slone RM, MacMillan M, Montgomery WJ. Spinal fixation. Part 3. Complications of spinal instrumentation. Radiographics.1993;13(4):797–816.
- Zampolin R, Erdfarb A, Miller T. Imaging of lumbar spine fusion. Neuroimaging Clin N Am. 2014;24(2):269-286.
- Togawa D, Bauer TW, Lieberman IH, et al. Histologic evaluation of human vertebral bodies after vertebral augmentation with polymethyl methacrylate. Spine. 2003;28(14):1521–1527.
- arreon LY, Glassman SD, Schwender JD, et al. Reliability and accuracy of fine-cut computed tomography scans to determine the status of anterior interbody fusions with metallic cages. Spine J. 2008;8(6):998–1002.
- Katayama Y, Matsuyama Y, Yoshihara H, et al. Clinical and radiographic outcomes of posterolateral lumbar spine fusion in humans using recombinant human bone morphogenetic protein-2: an average five year follow-up study. Int Orthop. 2008;33(4):1061–1067.
- Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int. 2013;4(Suppl 5):S392-S403.
- Hegde V, Meredith DS, Kepler CK, et al. Management of postoperative spinal infections. World J Orthop. 2012;3(11):182–189.
- Go JL, Rothman S, Prosper A, et al. Spine infections. Neuroimaging Clin N Am. 2012; 22(4):755–772.
- Lazennec JY, Fourniols E, Lenoir T, et al. Infections in the operated spine: update on risk management and therapeutic strategies. Orthop Traumatol Surg Res. 2011;97(6):S107–S116.
- Hayashi D, Roemer FW, Mian A, et al. Imaging features of postoperative complications after spinal surgery and instrumentation. AJR Am J Roentgenol. 2012;199(1):W123-W129.
- Hong SH, Choi JY, Lee JW, et al. MR imaging assessment of the spine: infection or an imitation? Radiographics. 2009;29(2):599-612.
- Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(3):iii11–24.
- Bommireddy R, Kamat A, Smith ET, et al. Magnetic resonance image findings in the early postoperative period after anterior cervical discectomy. Eur Spine J. 2007;16(1):27–31.
- Thakkar RS, Malloy JP, Thakkar SC, et al. Imaging the postoperative spine. Radiol Clin North Am. 2012;50(4):731–747.
- Van Goethem JW, Parizel PM, Jinkins JR. Review article: MRI of the postoperative lumbar spine. Neuroradiology.2002;44:723–739.
- Van Goethem JW, Van de Kelft E, Biltjes IGGM, et al. MRI after successful lumbar discectomy. Neuroradiology. 1996;38(1):S90-S96.
- Tali ET. Spinal infections. Eur J Radiol. 2004;50(2):120-133.
- Ledermann HP, Schweitzer ME, Morrison WB, et al. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228(2):506–514.
- Eguchi Y, Ohtori S, Yamashita M, et al. Diffusion magnetic resonance imaging to differentiate degenerative from infectious endplate abnormalities in the lumbar spine. Spine. 2011;36(3): E198–E202.
- De Maeseneer M, Lenchik L, Everaert H, et al. Evaluation of lower back pain with bone scintigraphy and SPECT. Radiographics. 1999;19(4):901-912.
- Love C, Patel M, Lonner BS, et al. Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med. 2000;25:963-977.
- Lin WY, Tsai SC, Chao TH, et al. Uptake of gallium-67 citrate in clean surgical incisions after colorectal surgery. Eur J Nucl Med. 2001;28(3):369-372.
- Palestro CJ, Kim CK, Swyer AJ, et al. Radionuclide diagnosis of vertebral osteomyelitis: indium-111-leukocyte and technetium-99m-methylene diphosphonate bone scintigraphy. J Nucl Med. 1991;32(10):1861-1865.
- Skanjeti A, Penna D, Douroukas A, et al. PET in the clinical work-up of patients with spondylodiscitis: a new tool for the clinician? Q J Nucl Med Mol Imaging. 2012;56(6):569-576.
- Fuster D, Tomás X, Mayoral M, et al. Prospective comparison of whole-body (18)F-FDG PET/CT and MRI of the spine in the diagnosis of haematogenous spondylodiscitis. Eur J Nucl Med Mol Imaging. 2015;42(2):264-271.
- Moritani T, Kim J, Capizzano AA, et al. Pyogenic and non-pyogenic spinal infections: emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. Br J Radiol. 2014;87(1041):20140011.
- Floris R, Spallone A, Aref TY, et al. Early postoperative MRI findings following surgery for herniated lumbar disc. Part II: a gadolinium-enhanced study. Acta Neurochir. 1997;139(12): 1101–1107.
- Hueftle MG, Modic MT, Ross JS, et al. Lumbar spine: postoperative MR imaging with Gd-DTPA. Radiology. 1988;167(3):817-824.
- Coskun E, Süzer T, Topuz O, et al. Relationships between epidural fibrosis, pain, disability, and psychological factors after lumbar disc surgery. Eur Spine J. 2000;9(3):218–223.
- Ross JS, Robertson JT, Frederickson RC, et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38(4):855–861.
- Bosscher HA, Heavner JE. Incidence and severity of epidural fibrosis after back surgery: an endoscopic study. Pain Pract. 2010;10(1):18–24.
Dr. Stechishin is a radiology resident and Dr. Vertinsky and Dr. Shewchuk are neuroradiologists with Vancouver General Hospital and the University of British Columbia, Vancouver, British Columbia, Canada; and Dr. Street is a spinal surgeon with the Vancouver Spine Surgery Institute and the University of British Columbia, Vancouver, British Columbia, Canada. The authors have no conflicts of interest to declare.
