AI-Based, Whole-Body Composition CT Quantification Software Gets FDA Nod
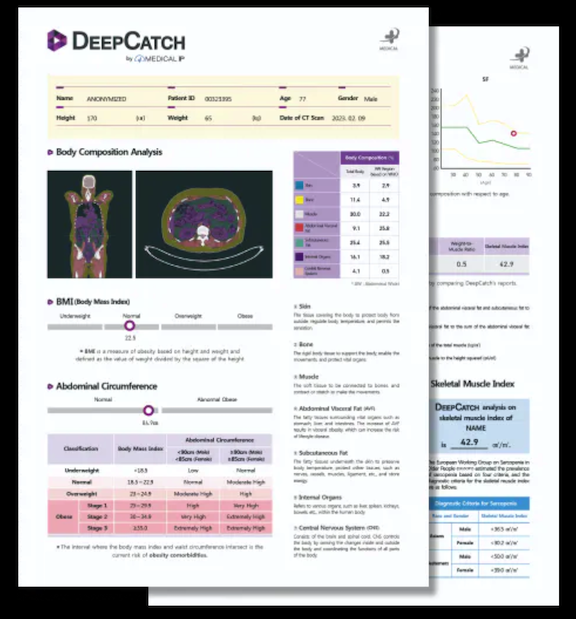
MEDICAL IP announced that its CT-based automatic body composition analysis AI software, DeepCatch, received US FDA 510(k) clearance. DeepCatch is a SaMD (Software as Medical Device) that automatically segments and analyzes anatomical structures in computed tomography
(CT) scan and provides the results as a report with 3D visual and quantitative information. DeepCatch is the only FDA cleared AI software that automatically analyzes various body components such as skin, bone, muscle, visceral fat, subcutaneous fat, internal organ, and central nervous system through whole-body CT.
In July 2022, a CPT code was issued for quantitative CT tissue characterization, including interpretation and report, obtained without concurrent CT examination of any structure contained in previously acquired diagnostic imaging. According to the company, this will provide an opportunity for DeepCatch, which calculates body composition information from CT, to be quickly introduced to the medical field in the US.
Medical staff will be able to provide additional screening for possible body composition-related diseases based on the DeepCatch report. Also, patients can obtain additional medical information about diseases such as obesity, metabolic diseases, sarcopenia, by using CT images taken during hospital treatment or health checkups. This makes it possible for anyone to manage their health efficiently and proactively.
The company also noted that during the FDA Clearance process, DeepCatch was tested for bias of various CT scanners, medical institutions, race, and ethnicity in a series of multi-national clinical trials, including in the US. As a result, the general-purpose performance and safety was confirmed through validation of the accuracy of AI-based analysis, including the measurement of the volume and area of body components and the body circumference.
DeepCatch can robustly derive clinically valid 3D body composition analysis results using CT, and, therefore, the company expects it will present a new standard for body composition.
MEDICAL IP CEO Joon S Park said, "There are various diseases related to body composition, such as geriatric diseases, cardiovascular diseases, and metabolic diseases such as diabetes and sarcopenia in the elderly and cancer patients, and DeepCatch is a product that can provide additional clinical information on these diseases. DeepCatch can be introduced to any medical institution around the world that takes CT scans, so with this FDA Clearance, we will be able to accelerate our strategy for global expansion."
