Mazabraud Syndrome
By Turan A, Unal TD, Hekimoglu A, Ozdemir M, Coşkun H, Sagra G


Case Summary
A middle-aged woman presented with a one-year history of a slow-growing and painless medial thigh mass. The patient had no history of trauma or primary malignancy. She had been on medication for hypertension for many years. There was no other medical history. A mobile mass in the middle of the leg was identified in the clinical examination.
Imaging Findings
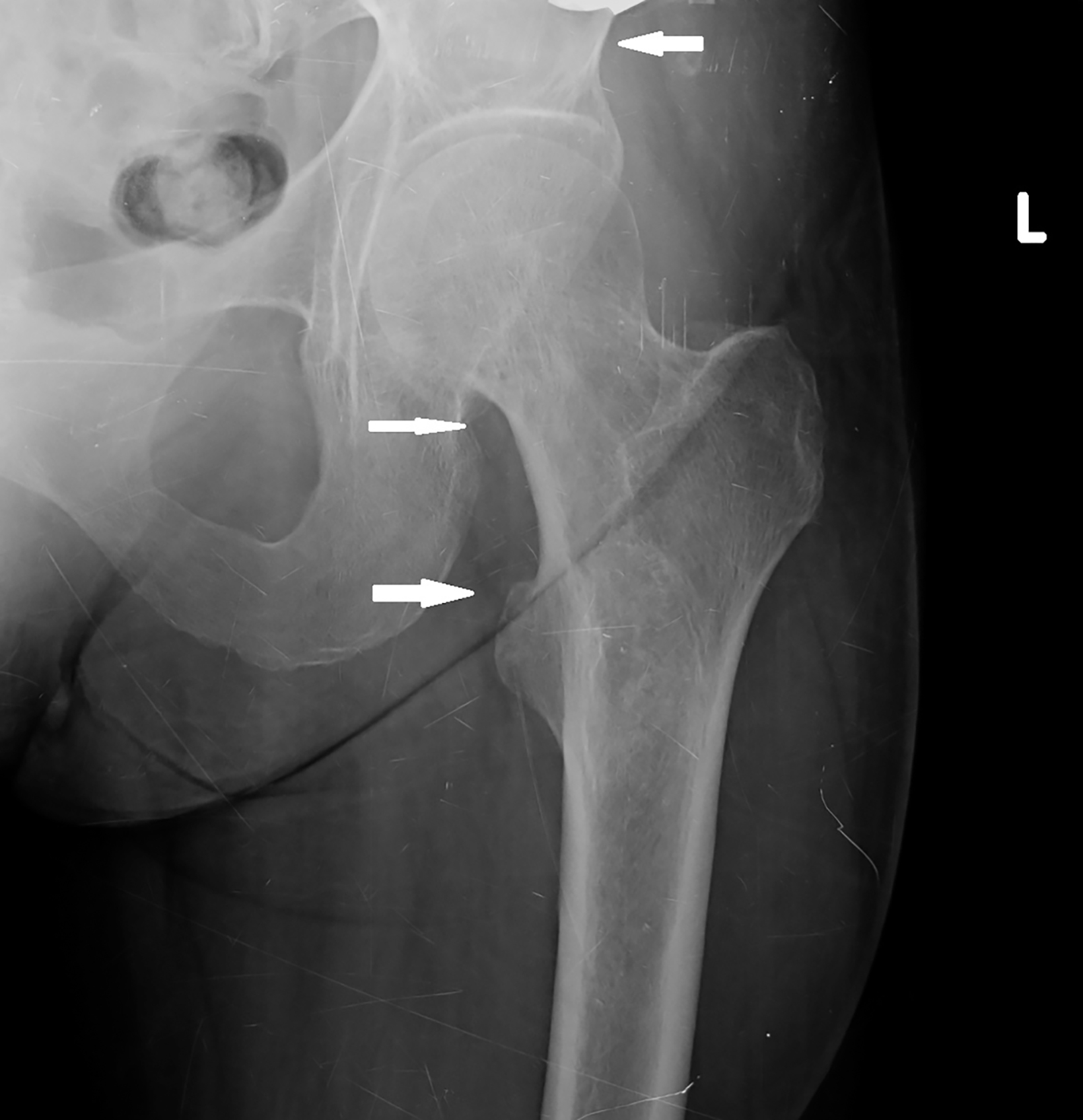
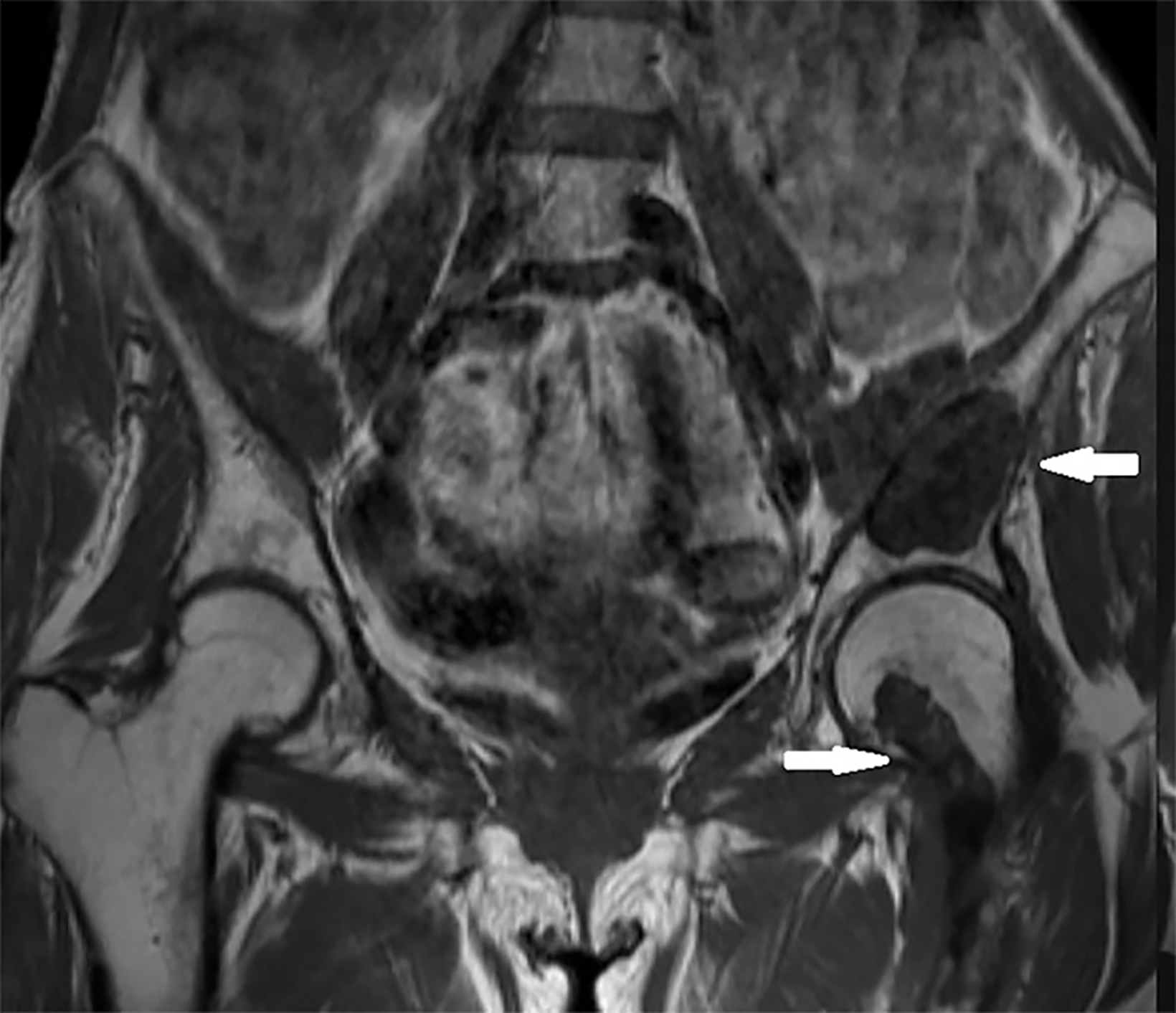
Initial radiographs of the left femur and hip showed well-defined, ground-glass lesions with sclerotic rims and no periosteal reaction (Figure 1). MRI of the left hip and thigh revealed a 5.6 × 3.1 × 3.0 cm lesion in the intramedullary area of the left iliac bone, causing slight bone expansion. The lesion was hypointense on T1 images, intermediate signal intensity on T2 images, and minimally enhanced following contrast administration. There was another lesion with similar imaging features in the left proximal femur. These bone lesions were diagnosed as polyostotic fibrous dysplasia (Figure 2).
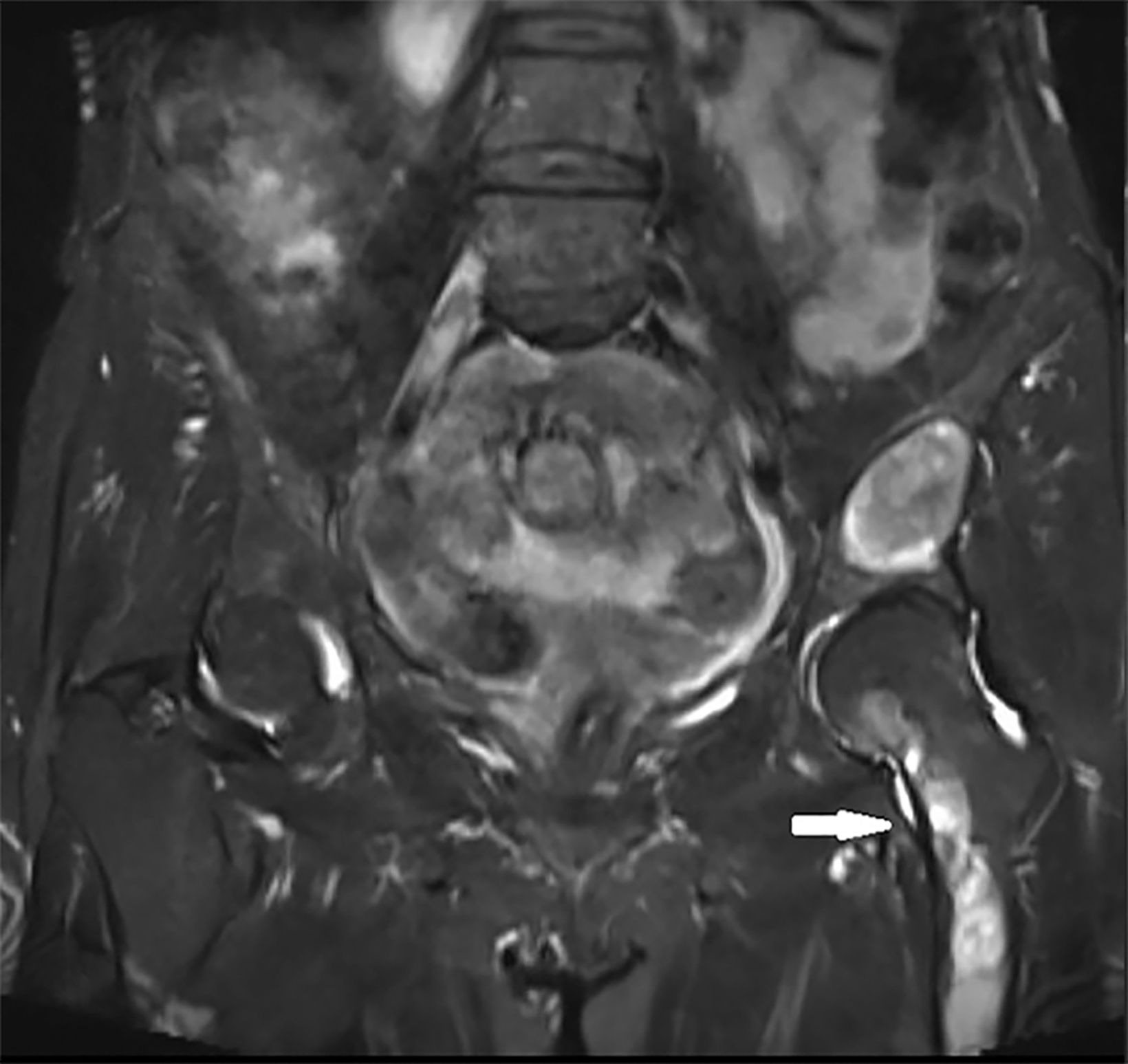
Ultrasonographic (US) findings of the clinically apparent soft tissue lesion showed a well-defined, mobile, heterogeneous, predominately hypoechoic mass. On MRI this measured 4.1 × 3.4 × 3.0 cm and was a well-defined, round, soft-tissue mass in the left vastus medialis muscle. The mass revealed prominent hypointense signal on T1 imaging, markedly hyperintense signal on T2 imaging compared to muscle, and minimal septal and peripheral enhancement following contrast administration (Figure 2). The MRI findings of the soft-tissue lesion led to myxoma as a diagnosis.
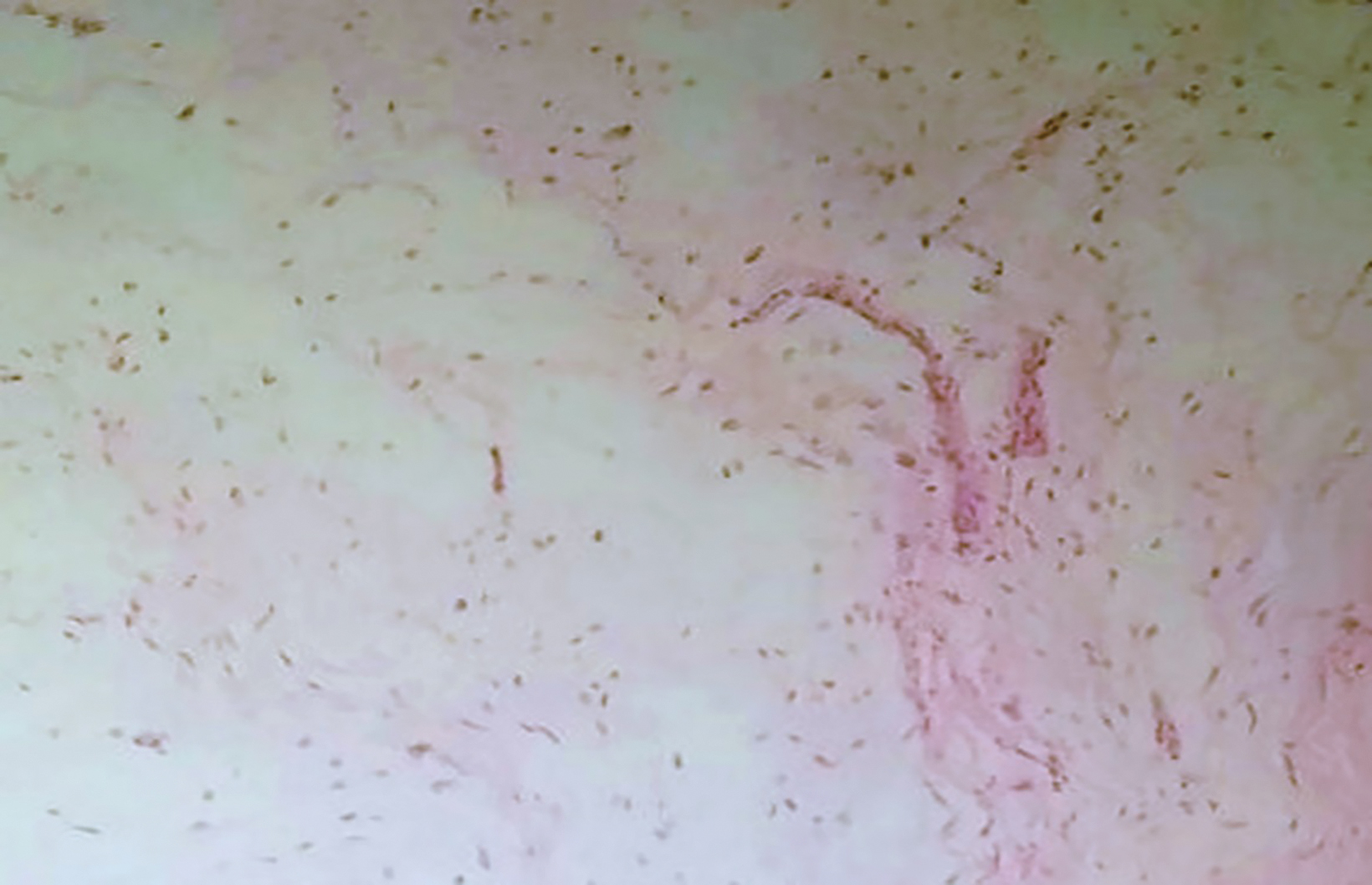
Surgical excision of the soft-tissue mass showed a well-circumscribed soft-tissue mass measuring 5 × 4.3 × 5 cm. Histologically, the lesion consisted of bland-appearing spindle cells within an abundant myxoid matrix. Hypocellular and hypovascular areas were surrounded by fine fibrocollagenous fibers. Atypical mitosis, nuclear pleomorphism, and necrosis were not observed (Figure 3). The lesion was histopathologically diagnosed as a myxoma.
Diagnosis
Mazabraud syndrome
Discussion
Mazabraud syndrome (MS) is a rare and complex disease that typically presents as the coexistence of fibrous dysplasia (FD) and intramuscular myxoma. This disease presents differently in children and adults.1 There is no evidence of genetic transition and it most commonly affects middle-aged females.2
The molecular etiology of MS is not entirely clear, but some genetic studies suggest that a mutation of Gsα protein encoded by GNAS-1 exists, and that this protein alters the level of cyclic adenosine monophosphate.3
Fibrous dysplasia is a congenital lesion of the bones. This osteoblastic maturation defect appears as a fibrous stroma and immature bone mold instead of normal bone. The monostotic form is more common; however, the polyostotic form is more highly associated with MS. It tends to affect the long bones, ribs, and cranium. It may cause pain, bone deformities, and pathological fractures.4
On radiography, FD is defined as an intramedullary lesion with cortical thinning and a sclerotic rim. A ground-glass appearance is typical on radiography and CT.1,4 On MRI, the lesions are iso-hypointense on T1 imaging, iso-hyperintense on T2, and show variable, heterogeneous contrast enhancement. It is reported that the sarcomatous transformation of FD is rare, occurring in less than 1% of cases.4 In the differential diagnosis of FD, other intramedullary bone lesions such as enchondroma, simple bone cyst, and medullary bone infarct may be considered.5
Myxoma presents as a slow-growing palpable mass which can be painful or painless. It is primarily located in the thigh, upper arm, calf, or buttock muscles. These are most often multiple in MS but sometimes can be solitary, as in our case.6
Myxoma is a primitive mesenchymal tumor that produces hyaluronic acid and immature collagen fibers. Because of the lack of mature collagen, it contains few stellate-shaped cells and rare vascular structures embedded in mucoid material. On US, it is seen as a hypo-anechoic mass
with well-defined margins and can show a microcystic appearance or contain internal echoes. It is hypovascular on color Doppler US.6
MRI typically demonstrates high signal intensity on T2WI and low signal intensity on T1WI. Lesions are commonly homogeneous and well-circumscribed. A thin rim of fat can be seen around the mass secondary to atrophy.1 In the perilesional region, fluid signal can be observed due to the leakage of myxoid material. There is a mild-moderate diffuse or peripheral-septal enhancement pattern.6
Myxoma should be differentiated from myxoid peripheral nerve sheath tumors, myxoid liposarcoma, and myxoid leiomyosarcoma. The perilesional fluid and thin rim of fat tissue around the myxoma are useful for differential diagnosis. Biopsy may not be required to establish the diagnosis of myxoma.1,6
Myxomas are always benign, and biopsy or surgical excision is generally not necessary.7 Fibrous dysplasia commonly shows slow progression; therefore, a conservative approach is preferable.2,5
Conclusion
Radiologists must be aware of the rarely seen solitary or multiple soft tissue lesions which are associated with FD. Knowing that the accompanying soft tissue mass is a myxoma will prevent unnecessary biopsy and resection. Following FD can provide early diagnosis in rare cases of malignant transformation.
References
- Case DB, Chapman CN Jr, Freeman JK, et al. Best cases from the AFIP: Atypical presentation of polyostotic fibrous dysplasia with myxoma (Mazabraud syndrome). Radiographics. 2010; 30(3):827-832.
- Fu S, Tian Z, Zhang C, et al. Monosotic fibrous dysplasia and solitary intramuscular myxoma of the head and neck: A unique presentation of Mazabraud’s syndrome and a literature review. Oncol Lett. 2015;10(5):3087-3094.
- Okamoto S, Hisaoka M, Ushijima M, et al. Activating gs(alpha) mutation in intramuscular myxomas with and without fibrous dysplasia of bone. Virchows Arch. 2000;437:133-137.
- Fitzpatrick KA, Taljanovic MS, Speer DP, et al. Imaging findings of fibrous dysplasia with histopathologic and intraoperative correlation. AJR Am J Roentgenol. 2004;182(6):1389-1398.
- Singnurkar A, Phancao JP, Chatha DS, et al. The appearance of Mazabraud’s syndrome on 18F-FDG PET/CT. Skeletal Radiol. 2007;36(11):1085-1089.
- Petscavage-Thomas JM, Walker EA, Logie CI, et al. Soft-tissue myxomatous lesions: review of salient imaging features with pathologic comparison. Radiographics. 2014;34(4):964-980.
- Munksgaard PS, Salkus G, Iyer VV, et al. Mazabraud’s syndrome: case report and literature review. Acta Radiol Short Rep. 2013;2 (4):1-4.
Affiliations: University of Health Sciences, Diskapi Yildirim Beyazit Health Application and Research Center, Radiology Dept., Ankara, Turkey (Drs Turan, Hekimoglu, Ozdemir, Koskun); University of Health Sciences, Kayseri Health Application and Research Center, Radiology Department, Kayseri, Turkey (Dr Unal); Ordu Medicalpark Hospital, Ordu, Turkey (Dr Sagra).
