Atrial septal aneurysm
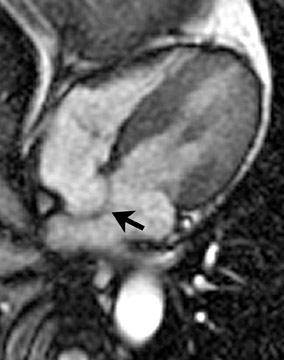

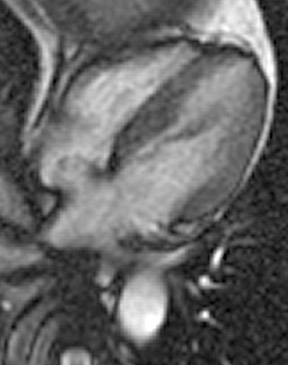
Atrial septal aneurysm
Findings
An electrocardiographic-gated segmented fast imaging employing steady state acquisition (FIESTA) cine sequence (repetition time 4.0, echotime 1.7) in the horizontal long-axis view was used to evaluate the atria. The MRI shows oscillation of the atrial septum into both atria throughout the cardiac cycle (Figure 1). The interatrial septum bulges into the left atrium at end-systole, moves to the midline at mid-diastole, and bulgesinto the right atrium at end-diastole. This is diagnostic of an atrial septal aneurysm (ASA). On MRI, the base of the aneurysm measured 1.9 cm. Maximum excursion into the right atrium was 1.3 cm and maximal excursion into the left atrium was 1.6 cm. No atrial thrombi were seen and no signal voids adjacent to the atrial septum were found to suggest the presence of a patent foramen ovale (PFO).
Discussion
Atrial septal aneurysm is a congenital cardiac abnormality that is characterized by saccular formation of the interatrial septum. Redundant atrial septal tissue results in bulging of the septum into either or both atria during the cardiac cycle. The diagnosis is best established with transesophageal echocardiography (TEE) since it can be easily missed with transthoracic echocardiography.1 Diagnostic criteria for ASA established by Hanley based on the appearance on echocardiography are: aneurysmal dilatation of the atrial septum protruding at least 1.5 cm beyond the plane of the atrial septum or phasic excursion of the interatrial septum during the cardiac cycle of at least 1.5 cm in total amplitude with a diameter at the base of the aneurysm of at least 1.5 cm.2 The condition can also be described using the Olivares-Reyes criteria, a classification based on the extent of excursion into each atrium.3 While the prevalence of ASA based on autopsystudies is <1%, the prevalence on TEE in nonselected patients is higher, in the range of 2% to 10%.3
Atrial septal aneurysm is clinically significant because of its association with cryptogenic stroke especially when there is a concurrent PFO. Approximately 70% of patients with ASA also have a PFO, while PFO is present in only 22% of patients without ASA. In one study, the combination of ASA and PFO was associated with a 33-fold higher risk of cryptogenic stroke.1 A meta-analysis has also found that ASA alone or ASA with PFO is associated with ischemic and cryptogenic strokes in patients <55 years of age. The strongest association is in those patients with both an ASA and a PFO.4 In patients <45 years of age with ischemic cerebral events, ASA should especially be suspected as a cardioembolic source. Mattioli and colleagues5 reported that 86% (43 of 50) of such patients had an ASA as the only possible etiology while 97% (42 of 43) of these younger patients with ASA also had a PFO.
Patients with both ASA and PFO who have had a stroke are at substantial risk for recurrent stroke despite aspirin therapy. Mas and colleagues6 reported that the risk for recurrent stroke was 15% for patients with both PFO and ASA compared with 4.2% for patients with neither abnormality. These patients might benefit from more aggressive therapeutic strategies such as combination antiplatelet drugs, long-term anticoagulation or closure of the PFO.6
Proposed mechanisms of stroke include formation of thrombus on the ASA, paradoxical embolism from a venous source through a PFO and thrombus formation from an atrial arrhythmia.7 Common maneuvers such as normal inspiration, coughing or Valsalva can result in transient elevation of right atrium pressures sufficient to permit paradoxical emboli to pass from the right atrium to the left atrium via a PFO.8It has been theorized that in patients with both ASA and PFO, motion of the fossa ovalis membrane may promote paradoxical shunting by enhancing the preferential orientation of blood flow from the inferior vena cava toward the foramen ovale.6 It has also been suggested that the thickness of an ASA is associated with risk of embolism. An ASA >5 mm in thickness has been found to have a higher association with stroke. The increased thickness may represent thrombotic material that has accumulated on the surface of the ASA.9 It is possible for an ASA to perforate, resulting in an interatrial communication that can place the patient at increased risk for paradoxical embolism.10
Other reports have also noted that ASAs are also associated with supraventricular tachyarrhythmias, mitral valve prolapse and migraines with aura.11-13 The association with arrhythmia, especially atrial fibrillation, would further increase the risk of stroke. The association with mitral valve prolapse is interesting, as it has been suggested that both abnormalities reflect redundancy of endocardial tissue.12 Up to 28% of patients who suffer from migraines with an aura have an ASA. One explanation for this relationship is that a PFO (associated with the ASA) would allow trigger substances for migraines (vasoactive chemicals) in the venous blood to bypass the pulmonary filter and enter the systemic circulation.14
On cine bright-blood MRI sequences, ASA can be easily diagnosed. The phasic excursion of the atrial septum throughout the cardiac cycleis readily apparent. If present, a signal void extending from the ASA into the left atrium would also indicate an associated PFO. Contrast-enhanced dynamic MRI with Valsalva has also recently been shown to be useful in the diagnosis of ASA and PFO.15 If echocardiography is inconclusive for the diagnosis of an ASA, MRI is the imaging study of choice for further evaluation.
CONCLUSION
Atrial septal aneurysm is a congenital cardiac abnormality characterized by oscillation and aneurysmal bulging of atrial septal tissue into either or both atria during the cardiac cycle. Roughly 70% of patients can also have a PFO, placing them at increased risk of cryptogenic stroke. Atrial septal aneurysm is easily recognized with cine bright-blood MRI sequences.
- Cabanes L, Mas JL, Cohen A, et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865-1873.
- Hanley PC, Tajik AJ, Hynes JK, et al. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: Report of 80 consecutive cases. J Am Coll Cardiol. 1985:6: 1370-1382.
- Olivares-Reyes A, Chan S, Lazar EJ, et al. Atrial septal aneurysms: A new classification in two-hundred five adults. J Am Soc Echocardiogr. 1997;10: 644-656.
- Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: A meta-analysis of case control studies. Neurology. 2000;55:1172-1179.
- Mattioli AV, Aquilina M, Oldani A, et al. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J. 2001;22:261-268.
- Mas J, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740-1746.
- Lamy C, Giannesini C, Zuber M, et al. Clinical and Imaging findings in cryptogenic stroke patients with and without patent foramen ovale: The PFO-ASA Study. Stroke. 2002;33:706-711.
- Wu LA, Malouf JF, Dearani JA, et al. Patent foramen ovale in cryptogenic stroke: Current understanding and management options. Arch Intern Med. 2004;164:950-956.
- Mattioli AV, Aquilina M, Oldani A, et al. Frequency of atrial septal aneurysm in patients with recent stroke: Preliminary results from a multicenter study. Clin Cardiol.2001;24:297-300.
- Ewert P, Berger F, Vogel M, et al. Morphology of perforated atrial septal aneurysm suitable for closure by transcatheter device placement. Heart. 2000;84:327-331.
- Morelli S, Voci P, Morabito G, et al. Atrial septal aneurysm and cardiac arrhythmias.Int J Cardiol. 1995;49:257-265.
- Belkin RN, Kisslo J. Atrial septal aneurysm: Recognition and clinical relevance. Am Heart J. 1990;120:948-957.
- Carerj S, Narborne MC, Zito C, et al. Prevalence of atrial septal aneurysm in patients with migraine: An echo cardiographic study. Headache. 2003;43:725-728.
- Wilmhurst PT, Nightingale S, Walsh KP, Morrison WL. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or strokeor for haemodynamic reasons. Lancet. 2000;356:1648-1651.
- Mohrs OK, Petersen SE, Erkapic D, et al. Diagnosis of patent foramen ovale using contrast-enhanced dynamic MRI: A pilot study. AJR Am J Roentgenol. 2005;184:234-240.
