Bariatric/Metabolic surgery for the radiologist — Part 1: Gastric restrictive surgery
Images
Editor’s note: This is the first installment of a two-part article. The second part will be published in the December 2016 issue of Applied Radiology.
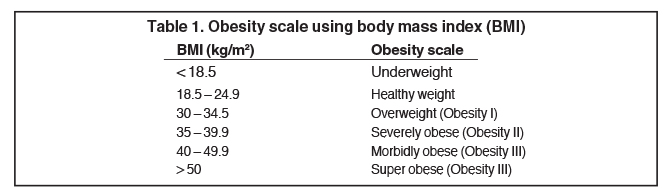
Approximately a third of American adults were clinically obese between 2007 and 2008, and the treatment of obesity-related medical conditions was estimated to cost almost $210 billion, accounting for more than 20% of U.S. national health expenditures.1 Obesity-associated conditions, including coronary artery disease, systemic and pulmonary hypertension, diabetes, osteoarthritis, hypercholesterolemia, cholelithiasis, respiratory problems and an increased incidence of endometrial, breast and colon cancer, lead to morbidity and mortality proportional to the degree of excess weight.2 The obesity scale using the body mass index (BMI) formula (BMI = weight (kg)/ height (m2 )) is shown in Table 1.
Bariatric procedures are indicated for morbidly obese adults (BMI > 40) or obese adults (BMI > 35) with an obesity related medical condition(s), who have failed behavioral and medical treatment.3,4 The procedure of choice is determined by each patient’s motivation, age, BMI and associated comorbidities after a multidisciplinary medical evaluation.3 Gastric restrictive procedures have gained favor over gastric bypass in the U.S. during the last 10 years. In 2008, a study of the previous 5 years showed an increase of laparoscopic adjustable gastric banding (LAGB) from 9% to 44% and laparoscopic sleeve gastrectomy (LSG) from 0.0% to 4%. In the same period, laparoscopic Roux-en-Y gastric bypass decreased from 85% to 51%.5 Greater than 50% excess weight loss (EWL) or other measurable health benefit, including resolution of sleep apnea and better control or cure of Type II diabetes mellitus and hypertension, define a successful surgical outcome. The EWL is approximately 50% for LSG and ranges from 23% to 70% for LAGB.6
Gastric restrictive surgery can result in complications common to any surgical procedure, such as bowel obstruction, infection with abscess formation, incisional/port hernia and pulmonary embolism. Complications specific to bariatric procedures are often diagnosed with upper GI fluoroscopy and CT scan and are summarized in Tables 2 and 3. This article will highlight the clinical features of gastric restrictive surgery relevant to the radiologist and review the radiologic appearance of normal and complicated LAGB and LSG.
Laparoscopic adjustable gastric banding (LAGB)
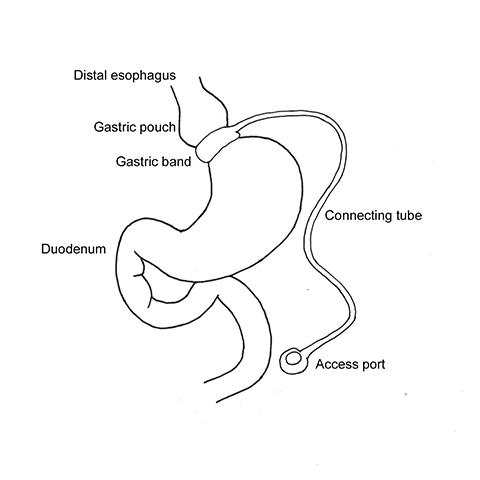
With LAGB, an adjustable, radiopaque silicone band is placed laparoscopically around the fundus of the stomach, creating a small gastric pouch that empties through a narrow stoma into the distal stomach (Figure 1). The gastric restriction aims to achieve early satiety, which will lead to decreased food intake.7-9 Advantages of LAGB over other procedures are reversibility, maintenance of normal gastrointestinal (GI) anatomy and less severe post-operative complications. LAGB has been shown to successfully control diabetes mellitus (>60%), hypertension (>40%) and hyperlipidemia (>50%).9 Inconsistent weight loss and the use of foreign material; ie, the silicone band, tubing and port are the main drawbacks of this operation.
The band is placed around the gastric fundus 2 cm below the esophagogastric junction without violating the lesser sac (pars flacida surgical technique). The band is secured with an anterior fundoplication of the gastric serosa to the pouch or metal barbs, which are present in some band models. Four to 6 weeks after the operation, the stoma size can be adjusted as needed by inflating the band with saline through the subcutaneous access port connected to the band via catheter.
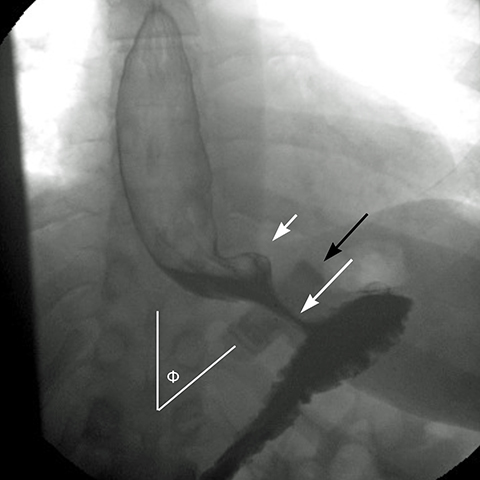
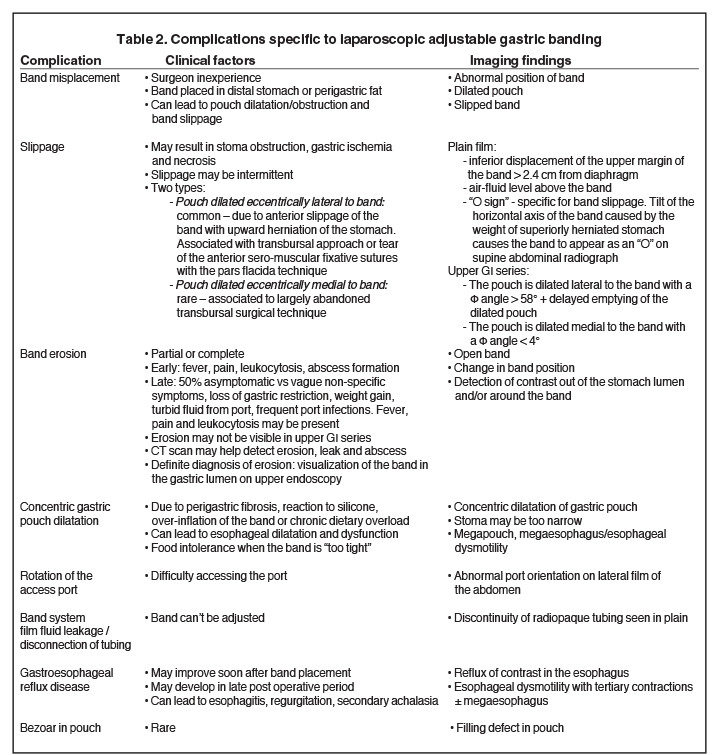
In an anterior-posterior (AP) projection, the band projects close to the diaphragm, inclined about 45° to the patient’s left. The normal Φ
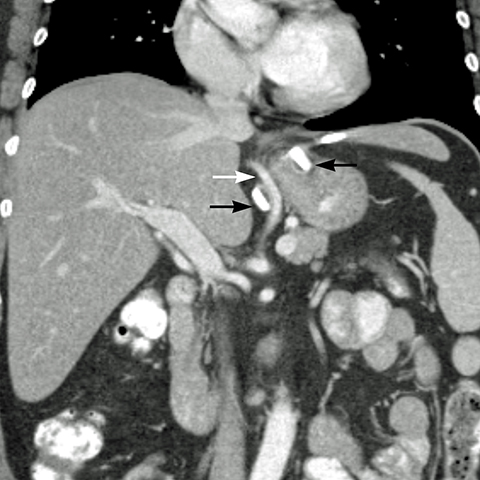
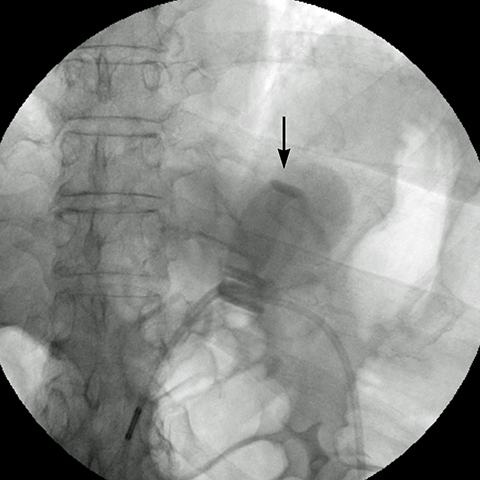
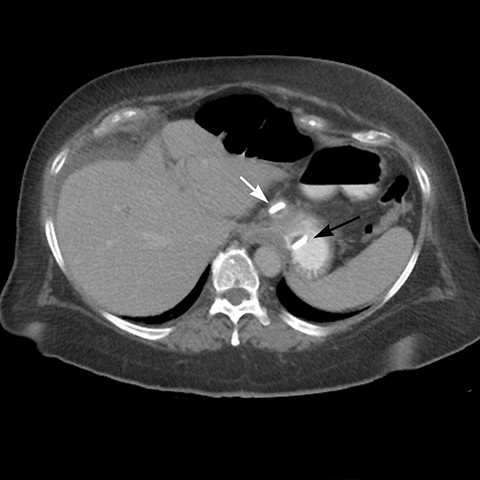
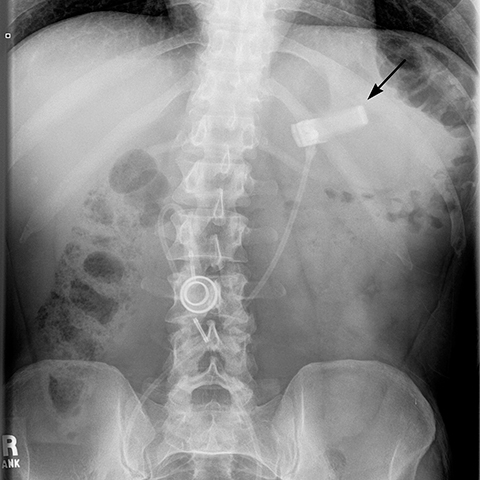
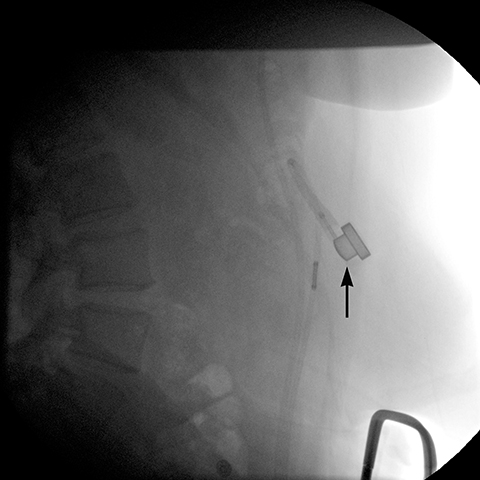
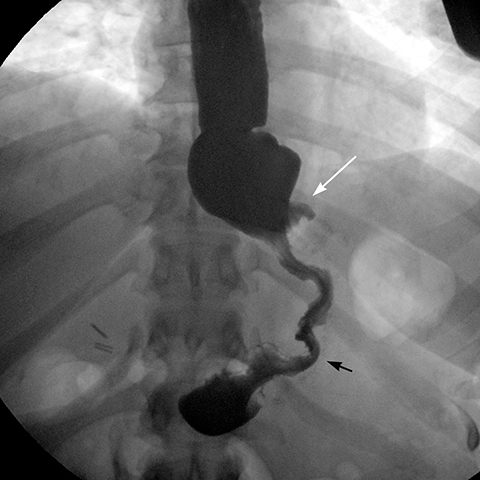
angle (the angle between the band and the spinal column) ranges 4–58°. The normal pouch capacity is 15-20 ml (approximately 4 cm in diameter) and the normal stoma diameter is 3 to 4 mm (Figure 2).7-9 Postoperative complications include band misplacement or slippage, infection, gastric band erosion, and pouch dilatation. The band may be misplaced in the perigastric fat or in the distal stomach. Misplacement in the distal stomach can lead to obstructive symptoms, pouch dilatation, and predisposes to slippage (Figure 3).
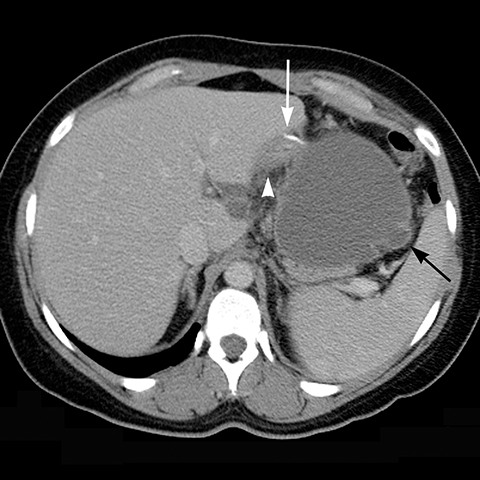
Early gastric band erosion usually presents with fever, pain and leukocytosis, and may result in an abdominal abscess. Suspected perforation and leakage are usually evaluated with upper GI series using water-soluble contrast. CT scan can demonstrate subtle leaks, which may not be seen in UGI series, and identify and guide drainage of an abscess.8
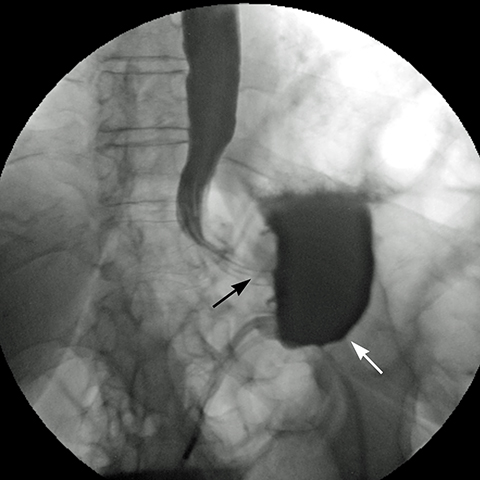
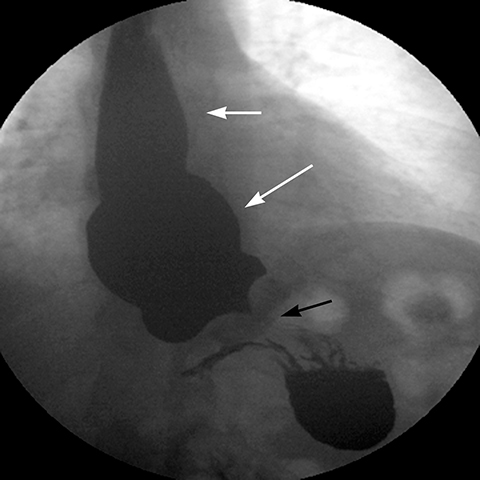
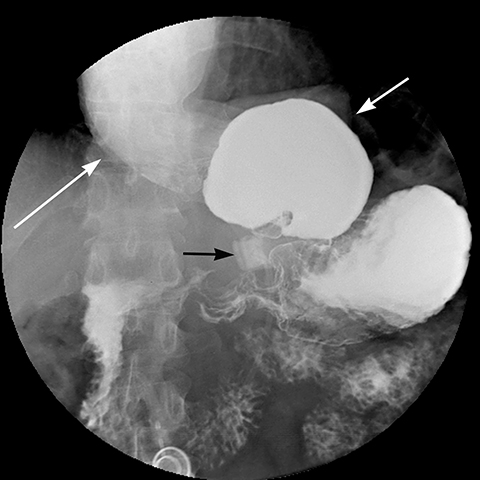
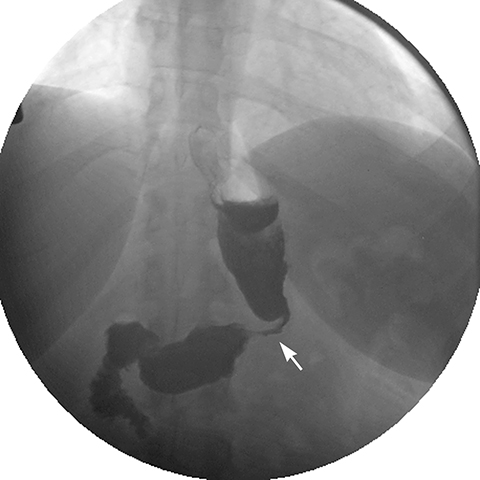
Late gastric band erosion occurs in 0.3 to 14% of patients9 with variable clinical presentations. Nearly half of patients are asymptomatic,10 while some may complain of loss of gastric restriction or weight gain, or there may be absence of fluid return or turbid fluid return from the port. Recurrent port infections may be a sign of band erosion. Intraoperative trauma to the gastric wall during band placement, inflammatory reaction to silicone, superimposed infection, and/or use of nonsteroidal anti-inflammatory medication have been implicated as possible etiologies. Erosion can be partial or complete. The imaging findings of erosion include an open band, change in band position and detection of contrast outside the lumen of the stomach and around the band (Figures 4 and 5). Partial erosion into the gastric wall may be difficult to detect on upper GI series. Definite diagnosis is made by visualization of the band in the gastric lumen during upper endoscopy. The treatment is removal of the band.
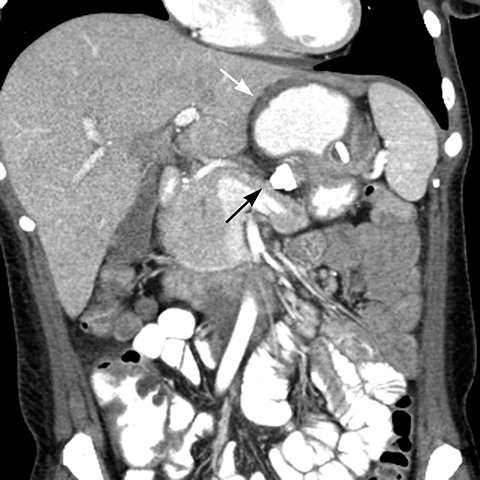
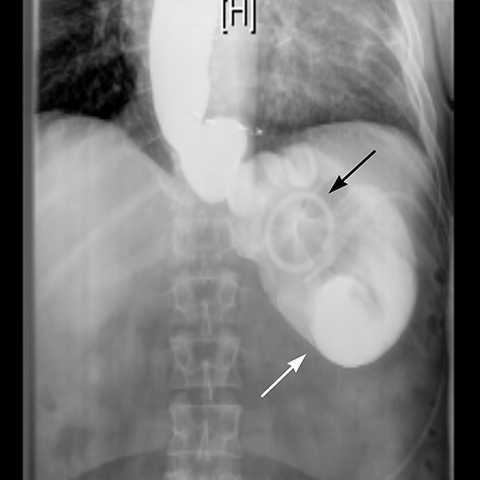
Gradual concentric distention of the pouch can be attributed to postsurgical perigastric fibrosis, a reaction to silicone, over-inflation of the band (Figure 6), or chronic dietary overload.7,8 Chronic pouch dilatation in the presence of a normal stoma due to dietary indiscretion is seen in 3 to 8% of patients and can result in a megapouch, esophageal dilatation and esophageal dysmotility (Figure 7).
Gastric band slippage is observed in 4% to 13% of patients11-13 and results in an eccentrically dilated pouch. In a rare type of slippage, related to a largely abandoned transbursal surgical technique, the pouch is eccentrically dilated medial to the band. The inferior and posterior parts of the stomach herniate and the band rotates counter-clockwise, resulting in a Φ angle < 4o . More commonly, tearing of the anterior fundoplication sutures can lead to anterior slippage of the band with upward herniation of the stomach.7,8 The gastric pouch is eccentrically dilated lateral to the band, the band rotates clockwise resulting in a Φ angle > 58o , and there is delayed emptying of the dilated pouch which may contain an air-fluid level (Figure 8).
Plain-film findings of slippage with high sensitivity and specificity include inferior displacement of the upper margin of the band by greater than 2.4 cm from the diaphragm (Figure 8) and air-fluid level above the band in the upright position.14 The “O sign” is specific for band slippage15 and refers to a circular, or O-shaped, configuration of the band seen in the supine AP radiograph of the abdomen, due to a tilt of the horizontal axis of the band caused by the weight of superiorly herniated stomach (Figure 9). Band slippage may result in obstruction of the stoma and cause gastric ischemia and necrosis. Rarely, slippage can result in gastric volvulus with ischemia.16 Intermittent band slippage, seen only when the pouch is full, may be a challenging diagnosis.
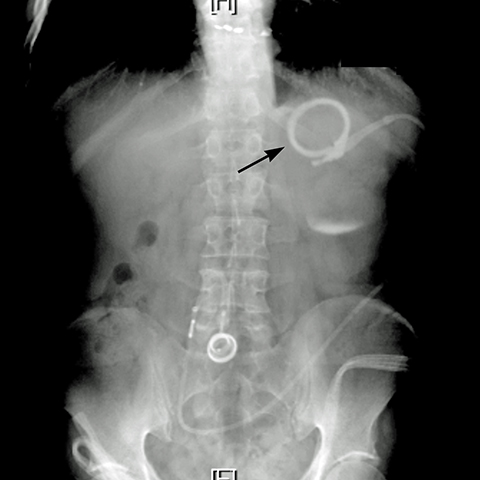
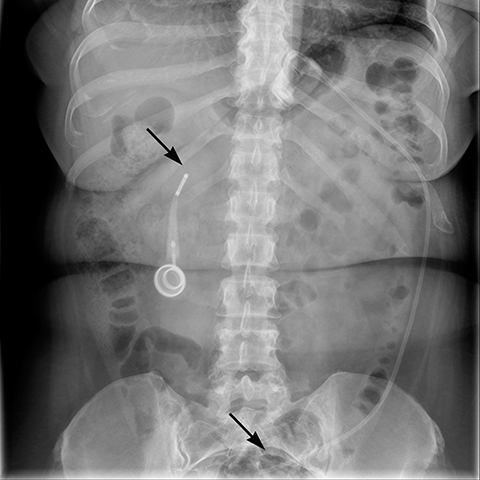
Additional complications include rotation of the access port (Figure 10), catheter detachment or breakage (Figure 11), fluid leak from the banding system, infection, trapping of food in the pouch, fibrosis along the band, bowel obstruction or erosion of adjacent tissue caused by the catheter.
Gastroesophageal reflux disease (GERD) can improve in the early postoperative period due to an anti-reflux mechanism provided by the band;17 however, pre-existing gastroesophageal sphincter insufficiency and inadequate dietary modification predispose to GERD in the late postoperative period. Esophageal complications include secondary achalasia, reflux with regurgitation, esophagitis, esophageal dysmotility and dilation.
Laparoscopic sleeve gastrectomy (LSG)
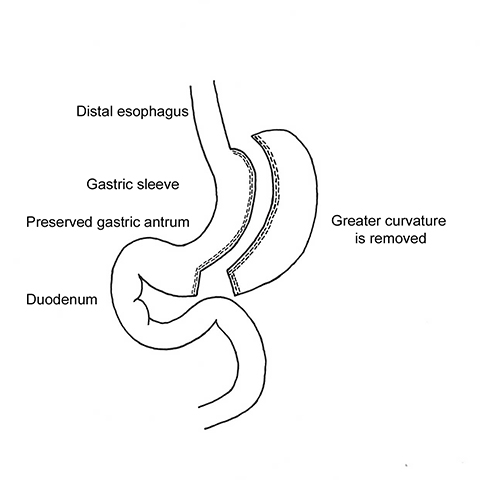
Laparoscopic sleeve gastrectomy (LSG) is a relatively new bariatric operation that is becoming increasing popular. Its advantages include adequate weight loss, avoidance of foreign material, excellent patient tolerance, ease of conversion to another bariatric procedure, decreased incidence of nutritional deficiencies and peptic ulcer disease, and preservation of normal gastro-intestinal continuity, which is suitable for endoscopic surveillance.6,17,18,20
In LSG, 75% of the stomach’s greater curvature is removed, resulting in a narrow, “banana-shaped” stomach with a capacity of 100 to 150 mL (Figure 1).21 The body of the stomach is divided with a staple-cutter device 3 to 6 cm proximal to the pylorus, at the level of the incisura angularis, preserving the antrum. A bougie (34–42 Fr) is used to guide sequential firings of the stapler to the angle of His. After the greater curvature is removed, continuous running sutures or buttress material applied over the staple-line help control hemorrhage and decrease adhesions. The two major advantages of LSG are removal of the elastic portion of the stomach and elimination of most of the ghrelin-producing portion of the stomach. The neuropeptide ghrelin controls the feeling of hunger and the distribution and rate of energy use in the body.6 LSG has a higher EWL and greater decrease in hunger sensation compared to LAGB, which can be attributed to the combination of gastric restriction and decreased post-operative serum ghrelin levels.17,22 Conversely, serum ghrelin levels remain unchanged or increase after LAGB.22
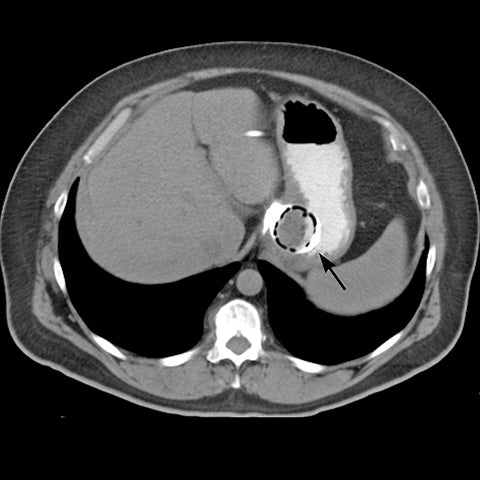
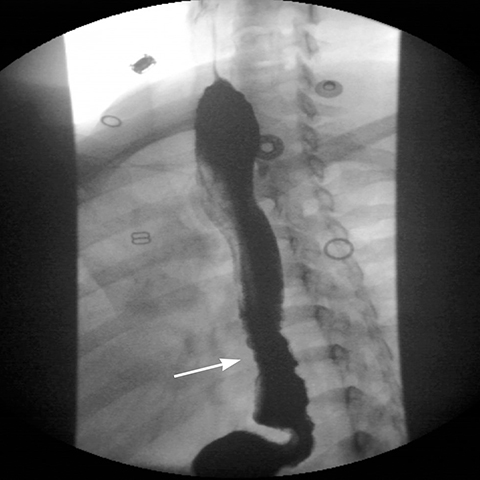
Early postoperative ileus after LSG presents with nausea and vomiting and the upper GI series will be normal23 or barium may pool in the proximal portion of the elongated stomach.21 Mechanical obstruction, most commonly at the incisura angularis, is associated with excessive narrowing from postoperative edema or scarring resulting in stricture (Figure 12). Endoscopic dilatation of a short stricture is often effective, while refractory long strictures may require surgical revision or conversion to Roux-en-Y gastric bypass.21,23
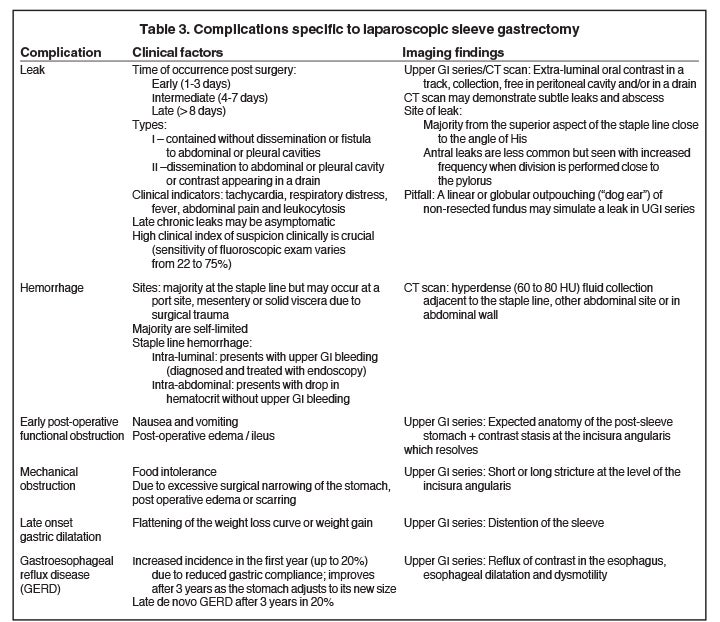
Staple line bleeding and leak are the two most common early complications of LSG. Contributing factors include poor healing of the suture line and decreased blood supply due to use of electrocautery for dissection along the greater curvature causing gastric wall heat ischemia.18,19
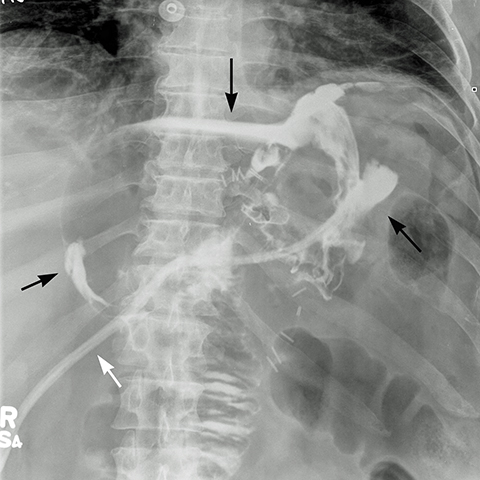
The incidence of leaks in LSG ranges from 0.7 to 5.3% (mean 2.3%).18 The clinical presentation varies from asymptomatic to peritonitis, septic shock, multi-organ failure and death. Leaks are classified chronologically as: early (1-3 days), intermediate (4-7 days) and late (> 8 days), and clinically as: (a) Type I – contained leak, with no dissemination or fistulae to abdominal or pleural cavities and lack of contrast material in the drain, and (b) Type II — dissemination to abdominal or pleural cavities, and/or with appearance of contrast material in the drain (Figure 13).18
Respiratory distress and tachycardia are the most reliable clinical indicators of a leak.18 Leaks may not be visible on the initial upper GI study, and appear on a subsequent study performed 4 to 28 days after surgery.24 The sensitivity of upper GI in diagnosing a leak ranges from 22 to 75%;23 therefore, a negative upper GI series should not dissuade surgical re-exploration or additional imaging when there is high index of clinical suspicion of a leak, given its high morbidity and mortality.23,25
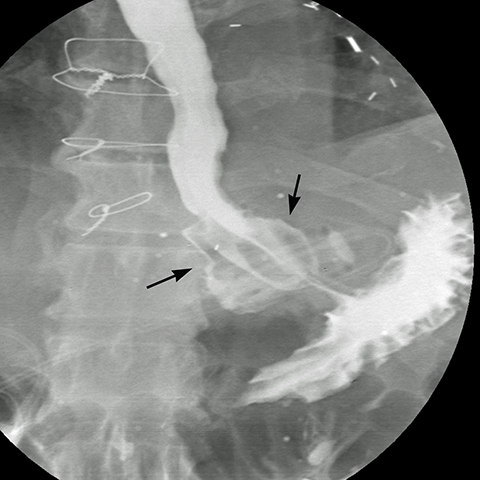
The majority of the leaks (85%) occur at the superior aspect of the staple line. Antral leaks are less common and seen when gastric division is done close to the pylorus where the gastric wall is thicker and more prone to dehisce.18 On upper GI series, extraluminal contrast is identified in a track, collection or free in the peritoneal cavity. If there is a high index of suspicion and water-soluble contrast upper GI series fails to detect a leak, a barium upper GI series or a CT scan may demonstrate a subtle leak and abscess.21 Linear or globular out-pouching from a residual portion of non-resected fundus may simulate a contained leak in UGI series (Figure 14).21 The remaining so called “dog ear” will facilitate surgical repair and avoid more extensive surgery in case a leak develops. The treatment of a leak depends on the time elapsed after the operation, its location and its magnitude. Early leaks generally need prompt surgical repair, while intermediate and late leaks may be managed clinically with placement enteral feeding tube, antibiotics, and CT guided drainage of abscess.19
Hemorrhage at the staple line may be intraluminal, presenting with hematemesis, and diagnosed on upper endoscopy. It may also be treated via endoscopic intervention. Intra-abdominal hemorrhage presents with drop in hematocrit without upper GI bleeding. Bleeding can occur at the staple line margin (Figure 15), at a port site, in the mesentery or in solid viscera due to surgical injury and can be diagnosed with CT scan. Most postoperative hemorrhages are self-limited and can be treated conservatively, but re-operation is required in hemodynamically unstable patients.23
Late onset gastric sleeve dilation results in flattening of the weight loss curve or weight regain and up to 4.5% of these patients may need reoperation.23 GERD increases in the first year after LSG and improves at 3 years after surgery, as the stomach adjusts to its reduced size. Still, late de novo GERD may appear after 3 years in 20% of patients with LSG, leading to esophagitis.17
Conclusion
Bariatric procedures are being increasingly performed to treat the growing population of obese American adults. Clinical insight to bariatric surgery and in-depth knowledge of postoperative imaging are indispensible for timely diagnosis and efficient management of complications.
References
- Cawley J, Meyerhoefer C. The medical care costs of obesity: An instrumental variables approach. J Health Econ. 2012; 31(1):219-230.
- Merkle EM, Hallowell PT, Crouse C, et al. Roux-en-Y gastric bypass for clinically severe obesity: normal appearance and spectrum of complications at imaging. Radiology. 2005; 234(3):674-683.
- Buchwald H. A bariatric surgery algorithm. Obes Surg. 2002; 12(6):733-746.
- Shah S, Shah V, Ahmed AR, et al. Imaging in bariatric surgery: Service set-up, post-operative anatomy and complications. Br J Radiol. 2011;84:101-111.
- Buchwald H ,Oien D. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009; 19(12):1605-1611.
- Gumbs AA, Gagner M, Dakin G, et al. Sleeve gastrectomy for morbid obesity. Obes Surg. 2007; 17(7):962-969.
- Mehanna MJ, Birjawi G, Moukaddam HA, et al. Complications of adjustable gastric banding, a radiological pictorial review. AJR Am J Roentgenol. 2006; 186(2):522-534.
- Prosch H, Tscherney R, Kriwanek S, et al. Radiographical imaging of the normal anatomy and complications after gastric banding. Br J Radiol. 2008; 81(969):753-757.
- Sonavane SK, Menias CO, Kantawala KP, et al. Laparoscopic adjustable gastric banding: What radiologists need to know. RadioGraphics. 2012;32 (4):1161-1178.
- Chisholm J, Kitan N, Toouli J, et al. Gastric band erosion in 63 cases: Endoscopic removal and rebanding evaluated. Obes Surg. 2011;21(11):1676-1681.
- Mortele KJ, Pattjnijn P, Mollet P, et al. The Swedish laparoscopic adjustable gastric banding for morbid obesity: Radiologic findings in 218 patients. AJR Am J Roentgenol. 2001;177(1):77-84.
- Blachar A, Blank A, Gavert N, et al. Laparoscopic adjustable gastric banding surgery for morbid obesity: imaging of normal anatomic features and postoperative gastrointestinal complications. AJR Am J Roentgenol. 2007;188(2):472-479.
- Zinzindohoue F, Chevallier JM, Douard R, et al. Laparoscopic gastric banding: A minimally invasive surgical treatment for morbid obesity. Prospective study of 500 consecutive patients. Ann Surg. 2003; 237(1):1-9.
- Swenson DW, Pietryga JA, Grand DJ, et al. Gastric band slippage: A case-controlled study comparing new and old radiographic sign of this important surgical complication. AJR Am J Roentgenol. 2014; 203(1):10-16.
- Pieroni S, Sommer EA, Hito R, et al. The “O” sign, a simple and helpful tool in the diagnosis of laparoscopic adjustable gastric band slippage. AJR Am J Roentgenol. 2010; 195(1):137-141.
- Kicska G, Levine MS, Raper SE, et al. Gastric volvulus after laparoscopic adjustable gastric banding for morbid obesity. AJR Am J Roentgenol. 2007; 189(6):1469-1472.
- Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: Results after 1 and 3 years. Obes Surg. 2006; 16(11):1450-1456.
- Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009; 19(12):1672-1677.
- Csendes A, Braghetto I, León P, et al. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg. 2010; 14(9):1343-1348.
- Braghetto I, Korn O, Valladares H, et al. Laparoscopic sleeve gastrectomy: Surgical technique, indications and clinical results. Obes Surg. 2007; 17(11):1442-1450.
- Levine MS, Carucci LR. Imaging of bariatric surgery: normal anatomy and postoperative complications. Radiology. 2014; 270(2):327-341.
- Kotidis EV, Koliakos G, Papavramidis TS, et al. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure? Obes Surg. 2006; 16(5):554-559.
- Herron D, Roohipour R. Complications of Roux-en-Y gastric bypass and sleeve gastrectomy. Abdom Imaging. 2012; 37(5):712-718.
- Carucci LR, Turner MA, Conklin RC, et al. Roux-en-Y gastric bypass surgery for morbid obesity: Evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology. 2006; 238:119-127.
- Gonzalez R, Sarr MG, Smith CD, et al. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007; 204(1):47-55.
Citation
FS M, EL W. Bariatric/Metabolic surgery for the radiologist — Part 1: Gastric restrictive surgery. Appl Radiol. 2016; (11):10-21.
November 2, 2016