Calcific cardioembolic infarction following aortic-valve replacement
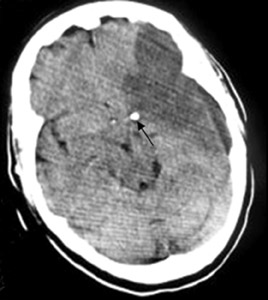
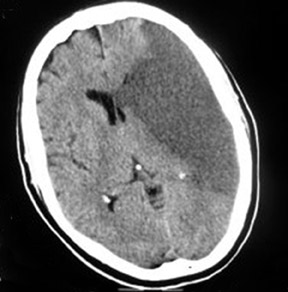
Middle cerebral-artery infarction from a calcific embolic material, following aortic valve surgery.
Findings
Computed tomography (CT) brain scans (pre- and postcontrast) showed a large calcific density in the proximal horizontal branch (M1) of the left-middle cerebral artery (MCA) with a large well-defined low-attenuation area in the left MCA vascular territory, accompanied by a small degree of midline shift to the right and distortion of the left lateral ventricle (Figures 1 and 2). This was consistent with an MCA infarct due to calcific embolism to the proximal M1 segment. The patient’s neurological condition rapidly deteriorated, and on the next day she was in a coma and died one day later. An autopsy was not performed.
Discussion
Calcific aortic valve stenosis is the most common acquired valvular-heart disorder found in developed countries and surgical intervention is often indicated.1
Calcified aortic valves can be a source of emboli to several regions of the body including the heart, limbs, retina and, rarely, the brain.2,3 Predisposing factors for calcific cerebral embolism include mechanical manipulation of valves during diagnostic or therapeutic procedures. Emboli may also occur spontaneously or rarely due to bacterial endocarditis.4,5
Twenty percent of patients with prosthetic heart valves will present with a cardioembolic stroke within 15 years of valve replacement,6 however, early stroke following aortic valve replacement, as in our case, is rare.
Neurological injury during and following coronary-artery or heart-valve surgery can rarely occur and is classified into 2 main groups.7,8 Type 1 injury (incidence 0.3% to 2%) includes all major and moderate focal injury (including coma and shock) due to major cerebral infarction. It typically results from emboli from an atherosclerotic ascending aorta, diseased carotid arteries, the prosthetic valve or the left cardiac chambers. Other rare potential causes of stroke following valve replacement include migration of the hemostatic sponge from the heart or distal metallic fragment embolization.9
Type 2 injuries are of lesser severity and/or duration. They include ophthalmologic complications, seizures, focal deficits of <24hours duration and cognitive and psychiatric disorders.8
The demonstration of a cerebral calcific embolus by CT scan was first documented by Yock in 1981.10 Although CT is generally less sensitive than magnetic resonance imaging (MRI) in identifying acute ischemic stroke, CT is more sensitive in detecting calcific cerebral emboli. This is especially illustrated in a recent case report where the calcific embolus was missed on MRI and the patient ended up having invasive angiography unnecessarily.5
Our case report therefore illustrates the utility of CT in detecting calcific cerebral embolism following aortic valve replacement. Though this type of stroke often occurs in closely monitored hospitalized patients, facilitating rapid diagnosis, management is difficult.
Thrombolysis with recombinant tissue plasminogen activator therapy may be contraindicated and may not work. This may relate to comorbidity and lesser likelihood of recanalisation of the blocked artery with conventional intravenous thrombolytic therapy.11 However,recently introduced intra-arterial mechanical devices for embolus entrapping and removal may potentially prevent any catastrophic embolic complication, and improve recanalisation rates and poor prognosis of those patients.12
The utility of CT in this clinical setting would therefore be even more evident allowing rapid treatment and potentially improving prognosis.
Conclusion
Our case report illustrates the utility of CT in detecting calcific cerebral embolism following aortic-valve replacement. With future developments in intra-arterial thrombectomy, rapid diagnosis and treatment of this rare entity could improve the final prognosis ofaffected patients.
- Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147.
- Eugleman D, Cohn L, Rizzo R. Incidence and predictors of transient ischemic attacks and strokes following coronary artery bypass grafting: Report and collective review. Heart Surg Forum. 1999; 2:242-245.
- Salka S, Almassi GH, Leitschuh ML. Spontaneous coronary artery embolus associated with calcific artery stenosis. Chest. 1994;105:1289–1290.
- Boon A, Lodder J, Cheriex E, Kessels F. Risk of Stroke in a cohort of 815 patients with calcification of the aortic valve with or without stenosis. Stroke. 1996;27:847-851.
- Gearry RB, Sharr JP, Avery SF. Spontaneous calcific cerebral embolus. Australas Radiol. 2005; 49:154–156.
- Thompson DB, Burton B, Watters M, et al. Calcific cerebral arterial embolization in the setting of bacterial endocarditis superimposed on prior rheumatic aortic valvular disease.Clin Imaging. 2003;27:304– 306.
- Ruel M, Masters RG, Rubens FD, et al. Late incidence and determinants of stroke after aortic and mitral valve replacement. Ann Thorac Surg. 2004; 78:77-83.
- Koullias G, Elefteriades J. In: Little A, ed. Complications in Cardiothoracic Surgery. Online: Blackwell, Futura; 2004:405.
- Gorriño O, Oleaga L, de la Fuente R, et al. Intracranial artifact in magnetic resonance caused by embolization of microscopic metallic fragment [in Spanish]. Neurologia. 2004;19:766-768.
- Yock DH Jr. CT demonstration of cerebral emboli. J Comput Assist Tomogr. 1981;5:190–196.
- Halloran JI, Bekavac I. Unsuccessful tissue plasminogen activator treatment of acute stroke caused by a calcific embolus. J Neuroimaging. 2004t;14:385-387.
- Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005;36:2311-2320.
