CT colonography: The essentials








Dr. Vandaele is a Resident in Radiology, University Hospital Gasthuisberg, Leuven, Belgium and a Visiting Fellow, Division of Abdominal Imaging and Intervention, Department of Radiology, Brigham and Women's Hospital, Boston, MA. Dr. Oliva is a Clinical Fellow, Division of Abdominal Imaging and Intervention, Department of Radiology, Brigham and Women's Hospital, Boston, MA. Dr. Barish is the Director of International Symposium on Virtual Colonoscopy, Boston, MA. He is also the Director of the 3D Imaging and Processing Center, and a Staff Radiologist, Division of Abdominal Imaging and Intervention, Brigham and Women's Hospital, and an Assistant Professor of Radiology, Harvard Medical School, Boston, MA. Dr. Mortelé is the Associate Director, Division of Abdominal Imaging and Intervention, and the Director, Abdominal and Pelvic MRI, Brigham and Women's Hospital, and an Associate Professor of Radiology, Harvard
Computed tomography (CT) colonography (virtual colonoscopy) is a promising new method for detecting colorectal polyps and cancers. Although multiple articles on this issue have been published since the mid-1990s, it remains an important discussion topic in current radiology and gastroenterology societies. Regarding its clinical role, there is no doubt that this imaging technique is best suited and highly recommended for those patients who are unable or unwilling to undergo conventional colonoscopy. Its role as a general screening tool for colon cancer is obvious for many, equivocal for some, and doubtful for others. This article is de-signed to highlight issues of importance for radiologists who are considering or who have recently started offering CT colonography to their patients and referring physicians.
Colorectal cancer is the second leading cause of cancer-related deaths in the United States. 1 The majority of colorectal cancers are believed to arise within benign adenomatous polyps and follow the adenoma-carcinoma sequence. The duration of this sequence is very long (±10 years), 2 and the removal of these precursor adenomatous polyps decreases the risk for lethal colorectal cancer significantly. 3
CT colonography uses multidetector-row CT to generate data, which is then converted by computer software into 2-dimensional (2D) and 3-dimensional (3D) displays of the colon. CT colonography has several advantages over conventional colonoscopy: No sedation is needed, it is only minimally invasive, and the examination is less time-consuming than conventional colonoscopy. However, there is still a need for bowel cleansing and insufflation of gas to expand the colon. Moreover, exposure to radiation is inherent to CT, and there is no possibility of biopsy, polypectomy, or treatment during the examination.
Patient preparation and examination
Optimal CT colonography technique requires careful cleansing and distention of the colon. Residual stool causes similar problems as those encountered with barium enema radiography, as it simulates polyps or masses. In theory, any preparation that results in a clean colon will suffice. Good colonic cleansing is achieved by a 24- to 48-hour low-residue diet and the ingestion of a cathartic or laxative that promotes evacuation of colonic contents. Saline cathartics, such as sodium phosphate (Fleet Phosphosoda, C.B. Fleet, Inc., Lynchburg, VA; VISICOL, Salix Pharmaceuticals, Inc., Morrisville, NC) or magnesium citrate (LoSo Prep, E-Z-EM, Inc., Lake Success, NY) are high-osmotic agents that induce increased peristalsis and evacuation. 4 These agents can be administered with bisacodyl (Dulcolax, Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT) the day before the examination. Dedicated preparation kits (Nutra-Prep, E-Z-EM, Inc., Lake Success, NY) exist as well. Such "dry preparations" are preferable to large volumes of electrolyte preparations commonly used for conventional colonoscopy, such as polyethylene glycol (Colopeg, Roche Laboratories, Gaillard, France; Colyte, Schwartz Pharma, Inc. Milwaukee, WI; GoLYTELY, Braintree Laboratories, Inc., Braintree, MA), be-cause with the latter, more residual fluid remains and retained fluid may obscure polyps at CT colonography. 5 Stool markers (mostly barium, Tagitol or TagitolV, E-Z-EM, Inc., Lake Success, NY) or iodine can be administered orally 24 to 48 hours prior to CT to improve differentiation of soft tissue intraluminal lesions and retained stool. This technique is called fecal tagging 6,7 (Figure 1).
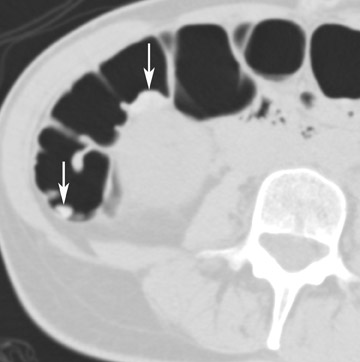
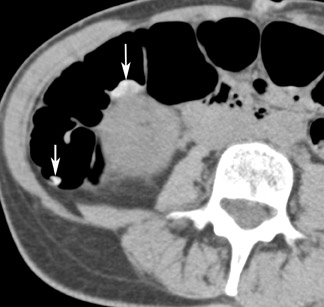
Colon insufflation is performed with the patient in a lateral decubitus position after placement of a catheter tip (with or without a retention balloon) in the rectum. The colon is insufflated with room air or CO 2 to maximum patient tolerance or to a set pressure. Therefore, an air bulb insufflator or an automatic inflation system can be used. Most patients will retain 1.5 to 2 liters of air. Carbon dioxide can be used instead of air, as it is absorbed by the colon mucosa and reduces discomfort. 4 The catheter is left in the rectum, and, with the patient in the supine position, a CT scout image is taken to confirm adequate colon distention (Figure 2). If adequate bowel distention is not achieved, additional air is insufflated into the colon. After scanning the patient in the supine position, the patient is placed in the prone position, and additional air or CO 2 is administered. Subsequently, CT is performed with the patient in the prone position. If a prone position is not possible for the patient, a left lateral decubitus position is preferred for optimal air and fluid redistribution. Several investigators have studied the benefit of the use of prone imaging in addition to supine imaging, and it has been shown to increase the sensitivity for detection of colonic polyps (Figure 3). 8-10
The usefulness of spasmolytic agents such as glucagon or Buscopan (Boehringer Ingelheim GmbH, Ingelheim, Germany) to improve bowel distention is controversial. 11,12 As a result of its effect on the ileocecal valve, it can cause unwanted reflux of air in the small bowel and may secondarily reduce colonic distention. Generally, no bowel relaxant is used. 4 Administration of intravenous contrast material can sometimes be helpful in the characterization of detected lesions, 13 but intravenous contrast is usually not used in the screening setting because of the side effects of iodinated contrast agents and the increased cost.
The current use of multidetector-row CT makes single-breath-hold acquisition possible. The examination is generally well tolerated by patients, and the lack of recovery time allows patients to return to their normal activities shortly after the procedure. 14
Technical scanning parameters and interpretation issues
The use of a multidetector-row CT scanner with narrow detector collimation is a prerequisite for CT colonography and optimal 3D reconstructions. 15 The CT parameters to use are dependent on the type of scanner, the image quality needed, and the desired radiation dose. Commonly used parameters are thin-detector collimation (0.6 to 2.5 mm), table speed 6 to 16 mm/sec, 120 to 140 kV, and low tube current ({10}-50-70-100 mAs).
Such relatively low CT tube currents can be used, as opposed tocomparison with conventional abdominal CT protocols (±200 mAs), because of the high inherent contrast between air and the colon wall. Images are reconstructed as 1.5- to 3-mm-thick sections with a reconstruction interval ranging between 0% and 60%. Coronal and sagittal multiplanar reconstructions (MPR) are routinely performed. 16-21
The radiation dose of a virtual colonoscopy (supine/prone scan) can get as low as 2 mSv for an ultra-low-dose CT colonography (10 mAs) 16 and up to approximately 8.8 mSv at higher mAs. The same accuracy can be obtained at an effective dose level of approximately 3.6 mSv, resulting in a substantial decrease of the theoretical or 3D orthographic lifetime risk of developing fatal radiation-induced cancer. 17
Three-dimensional endoluminal images are useful to confirm the presence of a lesion and to improve diagnostic confidence. Most CTmanufacturers have software for creating 3D endoluminal and multiplanar reformatted views. Several software companies also sell special applications for CT colonography. 22 Workstations equipped with such software display 2D and 3D images on a single interactive screen, and this promotes a quick 2D to 3D correlation. Most investigators use a primary 2D interpretation with "lumen tracking," starting from the rectum and following the course of the bowel from slice to slice to the cecum. 23,24 Primary 3D endoluminal assessment of the colon (endoluminal "fly-through") is less often performed, as it is more time-consuming and is more susceptible to pitfalls. Three-dimensional imaging is complementary to axial and multiplanar reformatted 2D images and should be used as an adjunct to confirm the 2D observation and improve the characterization of a noticed lesion. 25 However, with further advancements in computer processing workstations, it is possible that the primary 3D approach will reappear on the scene, as good results in a large study have been reported by Pickhardt. 19 It is imperative that the 2D images are reviewed by using lung window settings (approximately W: 1600, C: -400). In addition, soft tissue windows must be used for further characterization of suspected lesions as well in regions of suspected colonic collapse (differentiation between colon collapse and pathologic wall thickening with narrowing of the lumen) and when searching for extracolonic abnormalities.
Interpretation of colorectal mucosal lesions
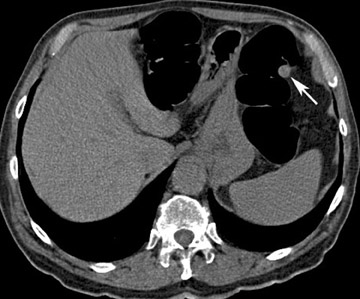
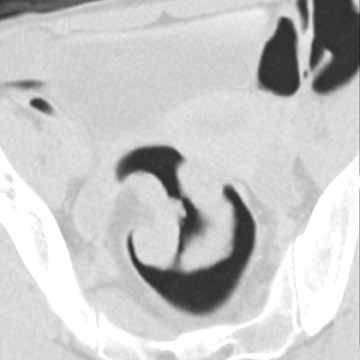
Every intraluminal projection in the colon requires careful analysis to determine whether it can be ignored or needs endoscopic confirmation and/or removal. With any imaging study, it is very difficult-if not impossible-to determine the histologic nature of a lesion. Therefore, radiologists must try to distinguish benign features from moderately and highly suspicious lesions through analysis of lesion characteristics. An important factor in the evaluation of colon filling defects is size, as the potential risk of malignancy increases with size. Morphology and attenuation are also important criteria to evaluate. 23 A lipoma, for example, is a submucosal tumor for which a confident diagnosis of a benign lesion can be made because of the low attenuation of adipose tissue (Figure 4).
The lesions to detect
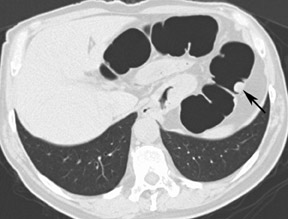
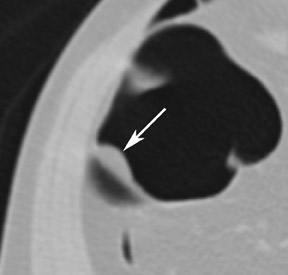
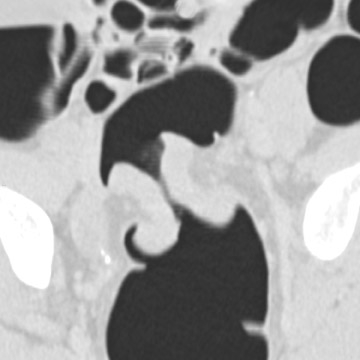
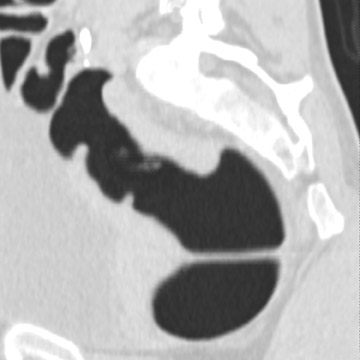
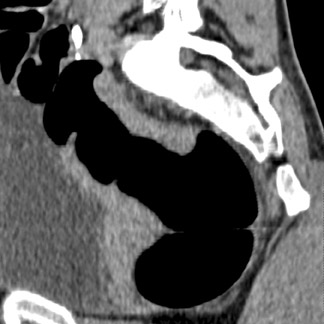
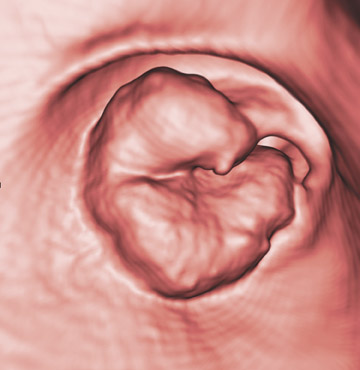
The aim of colorectal imaging and screening is to detect early malignancies or premalignant lesions. 2 Colorectal polyps appear as round, oval, or lobulated intraluminal projections and are homogeneous in attenuation. Every polyp should be measured and reported with its localization within the colon. When pedunculated polyps are measured, the diameter of the head should be measured. Most large colorectal carcinomas appear as fungating masses, often causing luminal narrowing (Figure 5). Changing from a colon window setting to a soft tissue window setting may facilitate identification of the lesion. Flat colorectal adenomas and carcinomas are difficult to identify, not only on 3D endoluminal images, but also on thin 2D images (Figure 6). 10 An isolated thickened fold with an irregular aspect and a different appearance from the adjacent folds may be due to an infiltrating tumor and should be noticed. Villous tumors often appear heterogeneous and, for less experienced readers, may mimic the heterogeneous aspect of stool.
Problems and pitfalls: How to overcome
The following potential pitfalls in the interpretation of CT colonography have to be considered: Fluid-filled segments and collapsed segments, retained stool, complex and bulbous folds, lipomas, foreign bodies, diverticulosis, the ileocecal valve, and extrinsic compression defects.
Fluid-filled segments and collapsed segments are often responsible for false-negative findings. The use of both supine and prone imaging will move colonic fluid and stool. The time delay between the 2 scans can be sufficient for transient segmental colonic spasm to resolve.
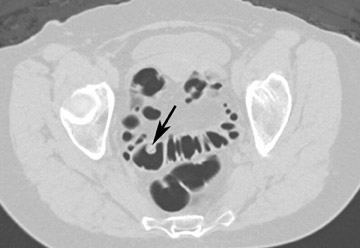
The vast majority of false-positive findings are related to residual fecal material. Therefore, the importance of a well-prepared colon cannot be over- emphasized. Several characteristics may help to differentiate stool from real lesions. Retained stool often has a heterogeneous aspect due to incorporated air or high-density food particles. The lack of wall attachment and enhancement, and changing location on prone/supine images suggests fecal origin (Figure 7). However, it is important to realize that some parts of the colon (the cecum, transverse colon, and sigmoid colon) are quite mobile because of the longer aspect of their mesentery. When observing mobile filling defects, a second entity to keep in mind is a pedunculated polyp with a long stalk. 10 The morphology of the polyp with a head and stalk is helpful in differentiating these lesions from mobile fecal material. Fecal material can be round, oval, or lobulated, which may mimic polyps, but often presents with angled borders and irregular geometric morphology. 23 Fecal tagging also helps to differentiate immobile retained stool from real lesions. 6,7
Irregular, complex, or bulbous interhaustral folds may appear as suspicious filling defects on axial images, especially in bowel segments that are not well distended. Visualization of the linear morphology of the fold on coronal, sagittal, and/or 3D endoluminal images can often solve the problem. The inner contour of a flexure also necessitates careful inspection, as small lesions are easily missed in this location.
Extrinsic compressions on the colon, which are potentially confusing at 3D endoluminal imaging, are easily characterized on 2D imaging, with adapted window settings.
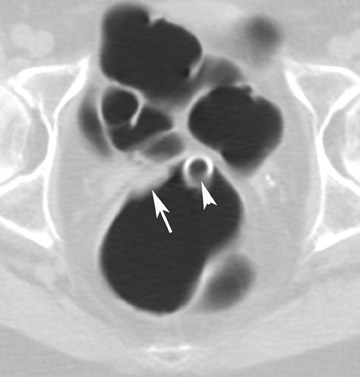
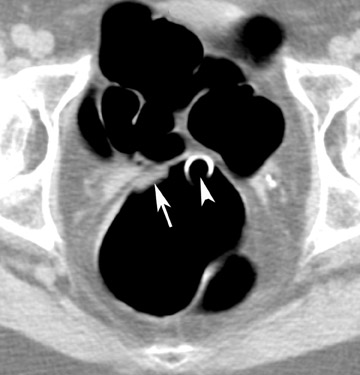
A common source of error is to mistake the ileocecal valve for a polypoid mass or vice versa. The ileocecal valve should be looked for in every patient. 26 Following the terminal ileum into the colon is helpful to confirm that the filling defect is the ileocecal valve. However, it is important to remember that the valve is covered by mucosa and that polyps may develop on the valve.
Validity/performance and accepted clinical role/value
The performance of virtual colonoscopy depends primarily on the size of the target lesion. CT colonoscopy has proven its diagnostic validity, especially in patients with incomplete conventional colonoscopy or with occlusive lesions in the distal colon, where virtual colonoscopy can identify synchronous neoplasms in the more proximal segments. 27,28
To succeed as an ideal screening test, a test should be sensitive, specific, safe, cost-effective, and acceptable to patients. There is still some debate about what constitutes a clinically significant colorectal polyp. 24,29 It is important to remember that adenomatous polyps (particularly advanced lesions) are the primary target of screening. 30 However, the rather low conversion rate from benign to malignant disease (only 3%) indicates that most individuals with adenomatous polyps will never develop colorectal carcinoma. 31,32 Since the prevalence of malignancy is extremely low in lesions smaller than 10 mm (only 1%), 33 this could be a good target. Patients with polyps larger than 10 mm should undergo an immediate colono-scopy for excision of the lesion. Diminutive filling defects (<5 mm) can represent normal mucosal protrusion, adherent fecal material, hyperplastic polyps, or small adenomatous polyps. Only the last has some potential for malignancy. The clinical significance of missing a diminutive adenoma is probably minimal, 34,35 and the question can be asked whether these very small polyps (<5 mm) should be reported at all. 24
What about lesions between 5 and 9 mm? This remains a dilemma. Most gastroenterologists remove these lesions when they are visualized endoscopically. However, when CT colonography is performed on an interval basis, missing these lesions is probably not important, since the adenoma-carcinoma sequence is very slow (up to 10 years). 24 Pickhardt et al 19 suggest 8 mm as a reasonable threshold for triggering immediate optical colonoscopy.
For screening purposes, guidelines must be set to stratify patients to undergo an immediate colonoscopy for polypectomy, short-term follow-up, or routine follow-up. Many data suggest that CT colonography has a high sensitivity and specificity for lesions >10 mm, which makes CT colonography a good candidate for a colon cancer screening test. 19,20,21,36,37 However, some recent publications have had less promising results, with poor sensitivity even for lesions >10 mm. 38,39 The reason for these poor results is probably multifactorial. Important factors on the general performance of CT colonography are colon preparation, scanning techniques, and reader experience. This stresses the need for adequate reader training before starting to perform CT colonography.
Extracolonic findings
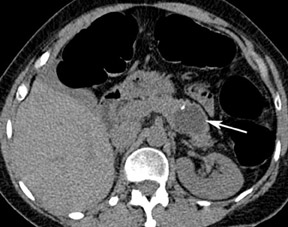
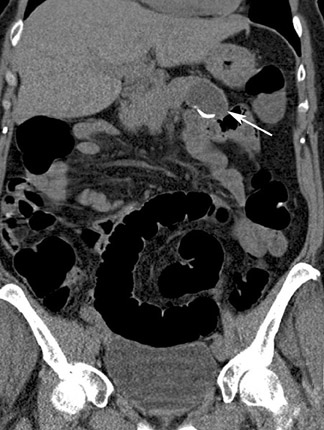
An important advantage of CT colon-ography in comparison with other colorectal examination tools is the ability to evaluate the extracolonic structures of the entire abdomen. CT colonography has the potential to save lives by detecting life-threatening lesions in a preclinical stage (Figure 8). A drawback is the economic impact of the supplementary cost of the further workup and treatment of these incidentally found lesions. The detection of equivocal disorders and potentially benign diseases induces often unnecessary patient anxiety. A distinction should be made in the interpretation of these extracolonic findings between highly important findings (requiring prompt intervention or further imaging workup), moderately important findings (generally benign, with the possibility of re-evaluation at a later time), and benign findings (no further evaluation nor treatment required). 40,41
Future applications and perspectives
Although great advances have been made, there are still some barriers to CT colonoscopy becoming a generally accepted technique for screening. These include a patient's reluctance to undergo colon preparation, colon insufflation, limited accuracy for small lesions, radiation dose, and the time required for interpretation of all the data. Research is ongoing in several domains to overcome these limitations.
Electronic cleansing by the digital removal of residual fluid, opacified after the administration of oral contrast, allows evaluation of colonic mucosa that would have otherwise been obscured. 6 In the future, further development of computer algorithms may lead to a "prepless" colon and make CT colonography more acceptable to patients.
Novel image display systems, such as colon unfolding techniques and computer-aided diagnosis algorithms (eg, automated polyp detection) may help to shorten interpretation time and may, perhaps, increase accuracy. 42-44
Research continues on ways to reduce the radiation dose to the patient, but radiation remains an inherent problem of CT. MR colonography is still, at least for the moment, in the experimental phase. 45-47
Conclusion
The optimal situation for interpretation of CT colonography is a clean and well-distended colon. A variety of filling defects may be encountered in the colon, and these should be further differentiated by the optimal use of 2D and 3D imaging techniques as well as the size, morphology, and attenuation characteristics of the defect. An experienced reader should be able to provide a clinically relevant recommendation (the need for colonoscopy or not) for the vast majority of filling defects as well for the extracolonic incidental findings. The feasibility of performing CT colonography in the unprepared colon and with the aid of computer-assisted techniques in the diagnosis may offer greater potential for this already exciting examination.
