FDA clears new 3D mammography solution
May 22, 2013 - Images generated using the C-View 2D imaging software can now be used in place of the conventional 2-dimensional (2D) exposure.
Hologic announced that the U.S. Food and Drug Administration (FDA) approved the use of its new C-View 2D imaging software. C-View 2D images can replace conventional 2D exposure previously required as part of a Hologic 3-dimensional (3D) mammography (breast tomosynthesis) screening exam.
C-View images are generated from the 3D tomosynthesis data acquired during the mammography exam. The new software eliminates the need for additional 2D exposures in a 3D mammography exam. In addition, the software allows radiologists to visualize the breast in more detail than with 2D mammography alone.
Hologic said that the combination of its 3D mammography technology and C-View 2D images will result in less time under compression for better patient comfort and a smaller dose of radiation.
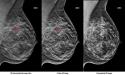
Image: C-View 2D images may be used in place of traditional 2-D images, eliminating the need for a separate 2-D exposure in a 3-D mammography exam. The images seen here show invasive ductal carcinoma (breast cancer). Image courtesy of Hologic.