Fresenius Kabi Launches New Brain MRI Contrast
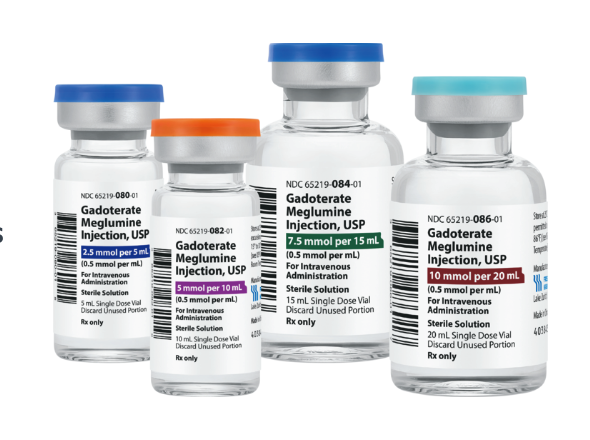 Gadoterate Meglumine Injection, USP, a new bioequivalent and therapeutic equivalent substitute for the contrast agent Dotarem, has been introduced by Fresenius Kabi. It’s the second contrast agent launched in 2022 by the company, following the release of Iodixanol Injection, USP in July during a nationwide shortage, and will be available in four different volume, single-dose vials.
Gadoterate Meglumine Injection, USP, a new bioequivalent and therapeutic equivalent substitute for the contrast agent Dotarem, has been introduced by Fresenius Kabi. It’s the second contrast agent launched in 2022 by the company, following the release of Iodixanol Injection, USP in July during a nationwide shortage, and will be available in four different volume, single-dose vials.
“Fresenius Kabi is pleased to expand our contrast agent portfolio and our support for the radiology community with the launch of Gadoterate Meglumine Injection, USP,” said John Ducker, president and CEO of Fresenius Kabi USA. “Contrast agents are vital to patient diagnosis, and the addition of Fresenius Kabi Gadoterate Meglumine will provide hospitals and clinics across the U.S. with more high-quality choices to support patient care.”
Gadoterate Meglumine Injection is a gadolinium-based contrast agent (GBCA) indicated for intravenous use with magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues in adult and pediatric patients (including term neonates) to detect and visualize areas with disruption of the blood brain barrier (BBB) and/or abnormal vascularity.1 In the United States, GBCAs are used in 30-45 percent of the approximately 40 million MRI procedures performed each year.2
As a macrocyclic ionic GBCA, Gadoterate Meglumine offers the strongest bond between the gadolinium atom and the chelate compared to other GBCAs. This greater stability means that the gadolinium has a higher likelihood to be excreted from the body as opposed to separating from the chelate and being retained in the body.
