Imaging of the biliary tree: Infection, inflammation and infiltration
By Walshe TM, McLean KA, Patel R, Chang SD, Harris AC





A multimodality imaging approach is often required for imaging of the biliary tract. The evaluation of adults presenting with biliary disease depends to a large extent on clinical symptoms of pain, presence and duration of jaundice, prior history of gallstones and any associated symptoms such as fever or weight loss.1, 2 Ultrasound, computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP) and ERCP all have a role in the diagnosis of biliary pathologies.
A wide spectrum of disorders affects the biliary tract; it includes calculous, infectious, inflammatory and neoplastic entities. The most common clinical presentations of biliary disease include obstructive jaundice, pain and sepsis.3 Biliary tract imaging is crucial in determining the location, etiology, and severity of the disorder and any complications thereof.4 Imaging also guides management of biliary tract disease and any resulting complications; for example, determining the most appropriate and suitable intervention required, eg, ERCP (endoscopic retrograde cholangiopancreatography), EUS (endoscopic ultrasound), radiological intervention, or surgery.2,4
The objective of this article is to review the indications for use of ultrasound, CT, MRCP and ERCP in biliary tract disease. The imaging features of common and uncommon infectious, inflammatory and neoplastic processes affecting the biliary tree, as well as the radiological management options, will be discussed.
Infection of the biliary tree
The most commonly encountered infection of the biliary tract is acute pyogenic cholangitis. Less common infections such as acute suppurative cholangitis, recurrent pyogenic cholangitis, infestation with parasitic organisms and biliary tract infections affecting immunocompromised individuals may have similar presenting features, but they tend to occur in specific patient groups.
Acute cholangitis
Acute cholangitis is an acute biliary bacterial infection, which typically occurs in the setting of obstruction. Charcot’s triad describes the classical clinical presentation of fever, pain and jaundice and if associated with shock and lethargy, the constellation of findings has been described as Reynold’s pentad. The most common underlying etiology, occurring in 80% of cases is obstruction of the common bile duct (CBD) by calculi. Other potential causes include strictures due to sclerosing cholangitis or malignancy and following instrumentation of the biliary tree. Factors known to increase the risk for developing cholangitis in patients with biliary calculi include: advanced age, neurologic disease, and periampullary diverticula. A number of life-threatening complications can result from acute cholangitis including: sepsis leading to multiorgan failure, hepatic abscesses, acute suppurative cholangitis, portal vein thrombosis and biliary peritonitis.5
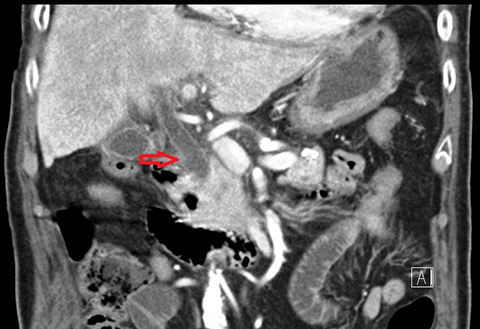
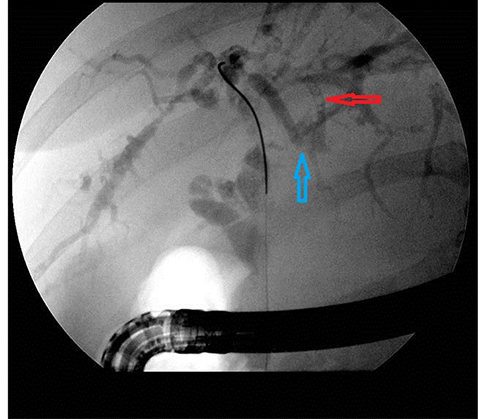
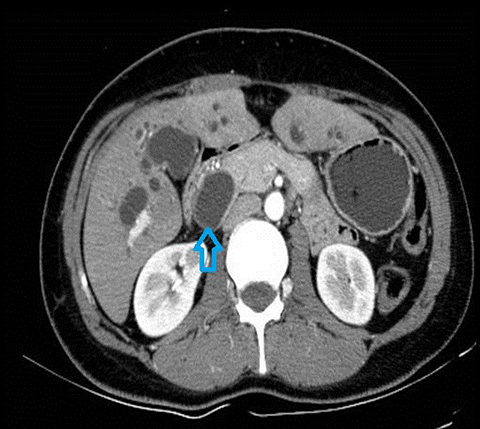
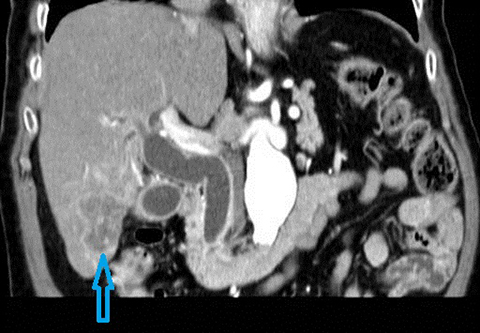
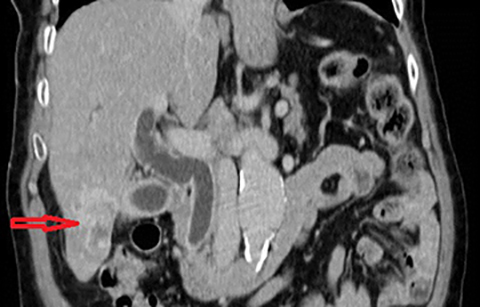
In acute cholangitis, the imaging findings include both biliary and parenchymal changes. Biliary changes include dilatation of the common bile duct in the setting of obstruction with dilatation of intrahepatic biliary ducts which can involve the central or segmental bile ducts only or diffusely involve the whole biliary tree (Figure 1). In 85% of cases, this is associated with smooth and symmetric extrahepatic bile duct wall thickening but less commonly, is associated with enhancement of the intrahepatic biliary duct walls (a finding best seen on delayed phase gadolinium-enhanced fat-saturation T1-weighted 3D gradient echo sequences).
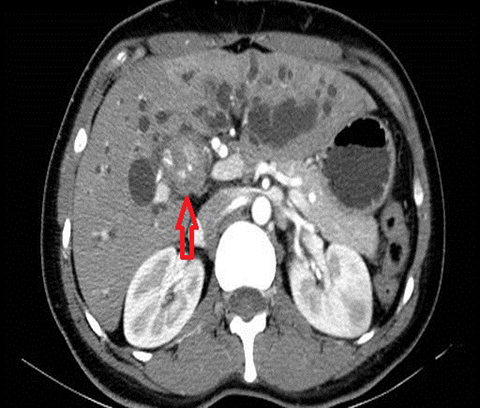
The parenchymal changes include increased T2 signal intensity (occurs in approximately 70% of cases) in a wedge-shaped or peri-biliary distribution (Figure 2). Hepatic parenchymal enhancement may be detected in the arterial phase only in approximately 60% of cases, in the delayed phase in approximately 15% of cases, or in both the arterial and delayed phases in approximately 36% of cases. The pattern of enhancement may be wedge-shaped, peripheral patchy, or peribiliary.4,5 Cross-sectional imaging is also useful for identifying the underlying cause for cholangitis (stone, stricture or neoplasm).
Acute suppurative cholangitis
Acute suppurative cholangitis is a life-threatening condition characterized by the presence of pus in the biliary tree occurring in up to 60% of cases of acute cholangitis. Emergency endoscopic or percutaneous biliary drainage is necessary for decompression.
The imaging findings suggestive of acute suppurative cholangitis are heterogeneous arterial parenchymal enhancement but more specifically enhancement, enlargement (>10mm), and bulging of the papilla (a finding that has a 60% sensitivity and 86% specificity for acute suppurative cholangitis). On MR, the presence of intraductal purulent material is indicative of acute suppurative cholangitis. This is detected as intraluminal low signal intensity on heavily T2-weighted images and/or intermediate signal intensity on fat-suppressed T1-weighted images.6 Ultrasound, CT and MRI can determine the underlying cause for cholangitis (stone, stricture or neoplasm) and detect complications such as portal vein thrombosis or hepatic abscesses.6,7
Recurrent pyogenic cholangitis (RPC)
Recurrent pyogenic cholangitis is a progressive disease characterized clinically by recurrent episodes of bacterial cholangitis. The etiology is uncertain but a possible role of chronic infestation with liver flukes or parasites such as Clonorchis sinesis, Opisthorchis viverrini or Ascaris lumbricoides has been postulated. Persistent inflammation results in bile duct fibrosis, which leads to bile stasis, strictures, pigment stone formation and subsequently, progressive obstruction and recurrent infections.5 Left untreated, complications such as liver abscesses, portal vein thrombosis, biliary strictures and intrahepatic stones can occur. Patients with recurrent pyogenic cholangitis have an increased risk of cholangiocarcinoma (5-18%) which tends to occur in the atrophied or heavily stone-burdened hepatic segments. The prevalence of recurrent pyogenic cholangitis is highest in Hong Kong and Southeast Asia, but the incidence in the Western world is increasing due to immigration. RPC is most common in rural populations and lower socioeconomic groups. The overall incidence in Asia is gradually decreasing, presumably related to improved living standards.8, 9
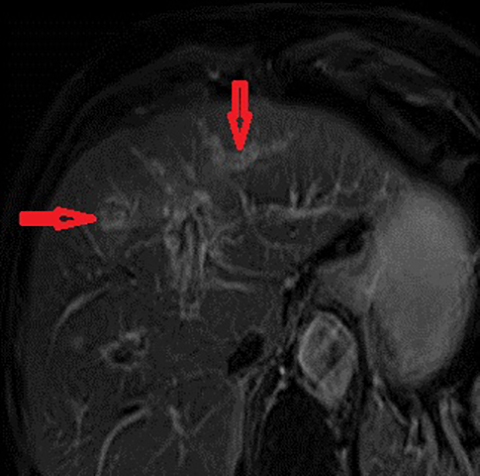
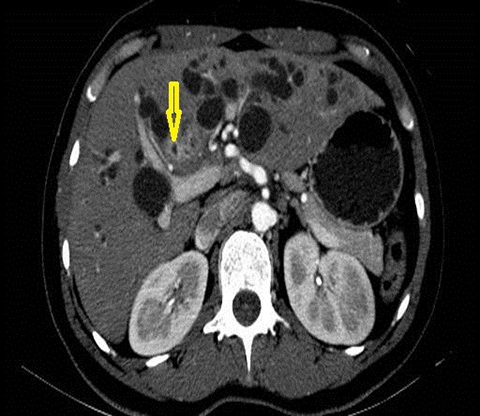
The imaging findings suggestive of RPC are intra- and extrahepatic biliary ductal dilatation, with relative sparing of peripheral ducts. Intraductal stones occur in up to 80% of patients. Large intraductal calculi are often detected in the absence of gallstones in the gallbladder. These intraductal calculi may appear T1-hyper and T2-hypointense relative to liver due to their high proteinaceous content.10 Pneumobilia is a common finding and is due to either the reflux of enteric gas from stone passage across the ampulla or due to infection with gas-forming organisms. Stenosis and/or strictures of peripheral biliary ducts occur with decreased branching and abrupt tapering resulting in an “arrowhead” appearance. Non-filling of biliary ductal segments due to strictures of intrahepatic ducts results in the “missing duct” sign described on ERCP and MRCP.11 Stenoses rarely affect the extrahepatic biliary tree. Parenchymal and biliary duct wall enhancement can be seen in acute exacerbations (Figure 3). Parenchymal atrophy occurs in the chronic stages predominantly affecting the lateral segments of the left lobe of the liver and posterior segments of the right lobe.5,12
Parasitic infections
A wide variety of parasites can result in biliary tract infection; these include fascioliasis, schistosomiasias, clonorchiasis, ascariasis and echinoccoccosis. The clinical manifestations of parasitic infections are variable and can mimic bacterial acute cholangitis. Parasitic infection should be suspected in patients who have recently travelled to endemic regions. Underlying infection may be identified by stool exam, serology and detection of eosinophilia. Imaging findings often include biliary dilatation and intra-biliary filling defects.5
Fasciola hepaticus is due to a trematode found in fresh water plants, occurring relatively commonly in developing countries. Infection due to Fasciola hepaticus is difficult to diagnose, until the advanced stage when the parasite is in biliary system. The known life cycle includes burrowing through the wall of duodenum, swimming to the right subphrenic space and burrowing through the liver capsule. Complications of this infection include hemorrhage and capsular retraction. Parenchymal imaging findings include subcapsular low attenuation lesions (so called “tunnels and caves”) and the only biliary finding may be periductal edema.13
Hepatic schistosomiasis infection is caused by the trematodes Schistosoma mansoni and japaonicum and has a high prevalence in Africa and Asia. Imaging findings include a coarse reticular pattern due to liver fibrosis, macronodular liver surface, septum-like fibrous bands extending to the liver surface with periportal fibrosis leading to cholestasis and ductal irregularity.14,15
Immunocompromised patients
Multiple biliary infections can occur in immunocompromised patients—for example, HIV-related cholangitis. The latter can have an imaging appearance similar to sclerosing cholangitis.16 Multiple biliary infections can occur in liver transplant recipients related to cholestasis from variety of causes such as chronic rejection, ischemia, drugs or anastomotic stricture and can result in the development of hepatic abscesses.5
Inflammation
Primary sclerosing cholangitis
Primary sclerosing cholangitis (PSC) is an idiopathic chronic cholestatic disease, which is presumed to be autoimmune in nature. It is characterized by diffuse cholangitis and progressive fibrosis of the biliary tree. PSC typically manifests in the 4th or 5th decade, with a male preponderance. There is a strong association with inflammatory bowel disease (60-80%), especially ulcerative colitis.17 One of the most concerning complications of PSC is cholangiocarcinoma which develops in up to 10% of patients, with an annual incidence of 0.6 - 1.5% per year. PSC patients develop cholangiocarcinoma two to three decades earlier than patients without risk factors. Despite this increased incidence, there are currently no evidence-based guidelines for screening for cholangiocarcinoma in patients with PSC, however, MRCP and measurement of CA 19-9 levels at annual intervals should be considered.
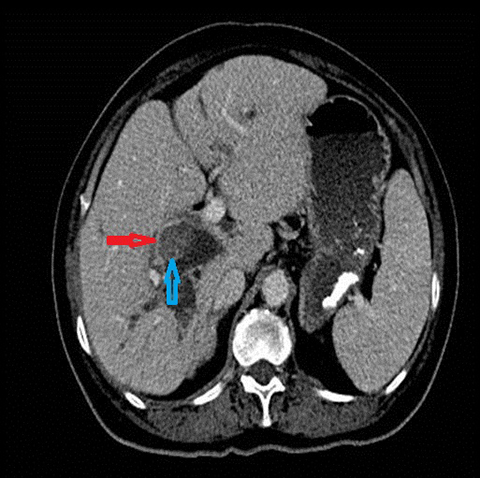
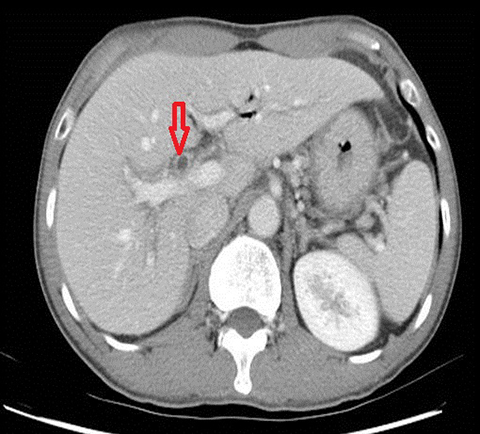
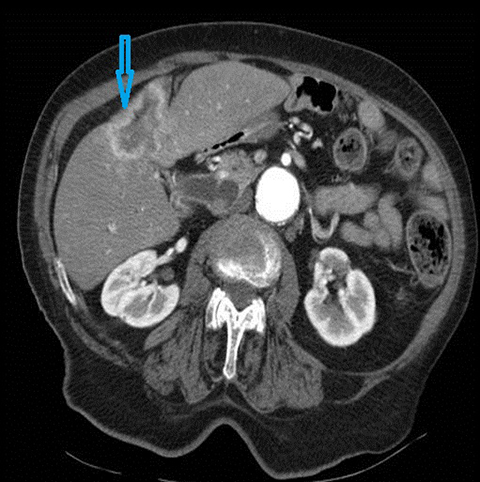
The classical imaging findings of PSC include multifocal strictures, segmental ectasia and irregular beading which typically involves both small and large ducts (75% of patients) but may be confined to small ducts (15%) or large ducts only (10%) (Figure 4). A feature that should raise the suspicion for superimposed cholangiocarcinoma is ductal wall thickening, greater than 4mm in depth.17, 18
Autoimmune pancreatitis-related cholangitis
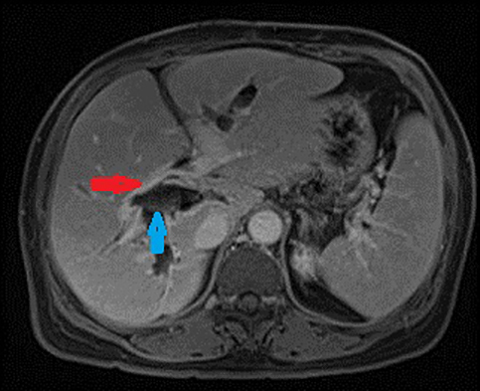
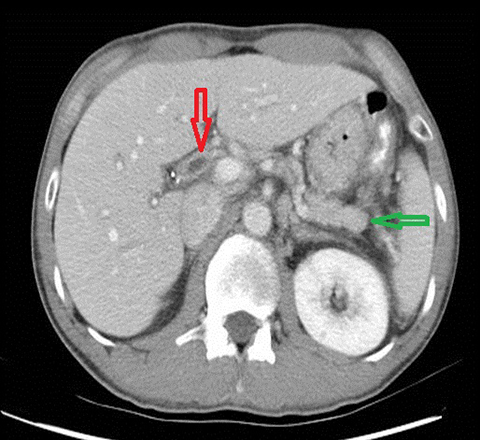
Autoimmune pancreatitis related cholangitis is an autoimmune systemic disorder characterized by infiltration of IgG4-positive lymphocytes into pancreatic or extra-pancreatic tissue. The classically described pancreatic imaging findings are diffuse parenchymal enlargement with featureless borders, decreased enhancement in the early pancreatic parenchymal phase following contrast administration and increased enhancement in the delayed phase.19 A common CT finding is a halo of soft tissue-attenuation surrounding the pancreatic parenchyma representing inflammatory cell infiltration.19 Biliary involvement is seen in up to 80% of patients with autoimmune pancreatitis. Both intra-and extrahepatic bile ducts may be involved, with the distal CBD most commonly affected. Multifocal bile duct strictures or mural thickening and enhancement are often seen and the appearance may mimic primary sclerosing cholangitis.20 (Figure 5). Gallbladder involvement with diffuse thickening of the gallbladder wall is increasingly recognized. The availability of histological tissue from a previous cholecystectomy can provide an important supportive clue in the diagnosis of autoimmune pancreatitis related cholangitis.21
Infiltration
Cholangiocarcinoma
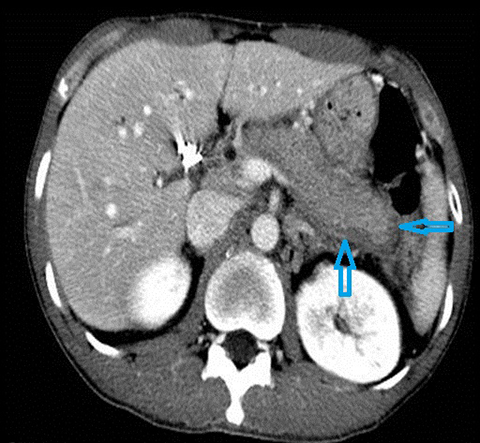
Cholangiocarcinoma (CC) is the second most common primary malignancy of the liver after hepatocellular carcinoma (HCC). Cholangiocarcinoma is an adenocarcinoma which arises from biliary duct epithelium often in the setting of pre-existing biliary tract disease such as PSC. The clinical presentation is typically with pain, a palpable mass, jaundice, weight loss and anorexia. Risk factors for the development of cholangiocarcinoma in addition to PSC include; recurrent pyogenic cholangitis, viral infections (Hepatitis B, Hepatitis C, EBV), biliary anomalies (eg. choledochal cysts, Figure 6), environmental or occupational toxins (eg, Thorotrast), alcohol consumption and smoking.22
Cholangiocarcinoma is classified based on the anatomic location into intrahepatic and perihilar and distal (extrahepatic) subtypes. From a treatment perspective, these different types are regarded as distinct entities. The intrahepatic type accounts for 10%, the perihilar subtype for 50-60% and distal subtype for 20% of all cholangiocarcinoma.22, 23 CC has also been classified on the basis of growth pattern into three types: (1) mass-forming exophytic type, which typically appears as a hepatic parenchymal mass; (2) periductal infiltrative type, (tumor growth progresses along the bile duct longitudinally) and (3) intraductal polypoid type which proliferates focally within the lumen of the diseased bile duct (least common, but most favorable prognosis).22-24
Intrahepatic cholangiocarcinoma are most often of the mass-forming exophytic type. The mass-forming subtype of intrahepatic cholangiocarcinoma typically presents with homogeneous attenuation mass with irregular but well-defined margins, irregular peripheral enhancement with gradual centripetal enhancement (Figure 7), associated capsular retraction and biliary duct dilatation at the tumor periphery. Vascular encasement is a common finding, usually without gross tumor thrombus.25 The differential diagnoses to consider in all patients with underlying liver disease is HCC with cirrhotic stroma, sclerosing HCC and the rare combined HCC-cholangiocarcinoma. Extrahepatic variants mostly infiltrate longitudinally along the bile ducts. Extrahepatic cholangiocarcinoma is most commonly perihilar (“Klatskin” tumor) in 50-60% of patients, or distal duct in location (20%) and less commonly multifocal (5%).23
Ultrasound is often the initial imaging modality used; it may detect features suggestive of cholangiocarcinoma, such as a heterogeneous, poorly defined mass, biliary dilatation or lobar atrophy. In PSC, US has a specificity and negative predictive value of 90%, but sensitivity and positive predictive value of only 50%. If contrast enhanced ultrasound is performed, washout of contrast from the lesion is typically detected.26
A multiphasic, contrast-enhanced CT is however necessary for diagnosis and staging. Non-contrast phase of imaging can facilitate differentiation between intraductal stones and tumor. The arterial and portal venous phases are useful for delineating the relationship of the tumor to arterial and venous anatomy and to facilitate surgical planning. Delayed enhancement of the tumor relative to liver parenchyma on delayed phase imaging is a characteristic finding of cholangiocarcinoma (occurring in approximately 70%). Dynamic contrast-enhanced MRI with MRCP is useful for detecting and characterizing the tumor as well as evaluating ductal extension or intraductal growth.4,24,25 Perihilar cholangiocarcinoma is classified using the Bismuth-Corlette classification system which taken together with information regarding vascular invasion, hepatic lobar atrophy and the presence/absence of metastases determines the potential for successful surgical resection.25
Biliary intraductal papillary mucinous neoplasm (IPMN)
Biliary intraductal papillary mucinous neoplasm has a number of synonyms including biliary intraductal papillary mucinous tumor, mucin-producing cholangiocarcinoma, mucinous ductal ectasia of biliary tree and mucin-hypersecreting carcinoma.
This tumor is characterized by intraluminal papillary masses which can result in biliary obstruction and dilatation. Some of these tumors secrete excessive mucin resulting in severe diffuse ductal dilatation. The clinical presentation is typically with intermittent abdominal pain, fever, nausea and jaundice. This tumor typically presents in the 5-7th decade in patients of eastern Asian ethnicity. The differential diagnosis includes cholangiocarcinoma, recurrent pyogenic cholangitis with stones and biliary cystadenoma/cystadenocarcinoma.27
MRI and MRCP are necessary in evaluating these tumors. The tumor is often small and flat or sometimes a fungating mass within dilated bile ducts. Very small or sessile tumors may be imperceptible on all imaging modalities. Mucin may be depicted on ERCP or PTC as multiple elongated linear, ovoid, or amorphous filling defects. The patterns of ductal dilatation vary and may be segmental or lobar due to tumor-induced obstruction, or aneurysmal. Multiple fungating tumors can cause generalized dilatation without visible tumor or generalized dilatation with greater dilatation of segmental ducts containing tumor.27
Conclusion
A wide range of pathologic entities affects the biliary tract, with cholangitis due to choledocholithiasis being the most common. However, other etiologies, including less common infectious processes, inflammatory conditions and neoplasia, should also be considered. A multimodality and multidisciplinary approach is often required for definitive diagnosis of biliary disorders and appropriate management.
References
- American College of Radiology Appropriateness Criteria ® on imaging of jaundice. http://www.acr.org/Quality-Safety/Appropriateness-Criteria.
- Yeh BM, Liu PS, Soto JA, et al. MR imaging and CT of the biliary tract. RadioGraphics. 2009; 29(6):1669-1688.
- Heller MT, Borhani AA, Furlan A, et al. Biliary strictures and masses: an expanded differential diagnosis. Abdom Imaging. 2014 Epub ahead of print.
- O’Conner O, O’Neill S, Maher MM. Imaging of biliary tract disease. AJR Am J Roentgenol. 2011; 197(4):W551-W558.
- Catalano OA, Sahani DV, Forcione DG, et al. Biliary infections: spectrum of imaging findings and management. RadioGraphics. 2009;29(7):
2059-2080. - Håkansson K, Ekberg O, Håkansson HO, et al. MR characteristics of acute cholangitis. Acta Radiol. 2002;43(2):175-179.
- Watanabe Y, Nagayama M, Okumura A, et al. MR imaging of acute biliary disorders. RadioGraphics. 2007;27(2): 477–495.
- Lo CM, Fan ST,Wong J. The changing epidemiology of recurrent pyogenic cholangitis. Hong Kong Med J. 1997; 3(3):302-304.
- Nakayama F, Soloway RD, Nakama T, et al. Hepatolithiasis in East Asia: retrospective study. Dig Dis Sci. 1986;31(1):21-26.
- Kim MJ, Cha SW, Mitchell DG, et al. MR imaging findings in recurrent pyogenic cholangitis. AJR Am J Roentgenol. 1999;173(6):1545–1549.
- Park MS, Yu JS, Kim KW, et al. Recurrent pyogenic cholangitis: comparison between MR cholangiography and direct cholangiography. Radiology. 2001;220(3):677-682.
- Heffernan EJ, Geoghegan T, Munk PL, et al. Recurrent pyogenic cholangitis: from imaging to intervention. AJR Am J Roentgenol. 2009 192:1;
28-35. - Dusak A, Onur MR, Cicek M, et al. Radiological imaging features of fasciola hepatica infection – a pictorial review. J Clin Imaging Sci. 2012; 2(1):1-8.
- Manzella A, Ohtomo K, Monzawa S, et al. Schistosomiasis of the liver. Abdom Imaging. 2008;33(2):144–150.
- Chou Y, Chiou H, Tiu C, et al. Duplex doppler ultrasound of hepatic schistosomiasis japonica: a study of 47 patients. Am J Trop Med Hyg. 2003; 68(1):18-23.
- Tonolini M, Bianco R. HIV-related/AIDS cholangiopathy: pictorial review with emphasis on MRCP findings and differential diagnosis. Clin Imaging. 2013;37(2):219-226.
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. AASLD Practice Guidelines. Hepatology. 2010; 51(2): 660-678.
- Menias CO, Surabhi VR, Prasad S, et al. Mimics of cholangiocarcinoma: spectrum of disease. RadioGraphics. 2009; 28(4): 1115-1129.
- Bodily KD, Takahashi N, Fletcher J, et al. Autoimmune pancreatitis: pancreatic and extrapancreatic imaging findings. AJR Am J Roentgenol. 2009; 192(2): 431-437.
- Eerens I, Vanbeckevoort D, Vansteenbergen W, et al. Autoimmune pancreatitis associated with primary sclerosing cholangitis: MR imaging findings. Eur Radiol. 2001;11(8):1401-1404.
- Leise MD, Smyrk TC, Takahashi N, et al. IgG4-associated cholecystitis: another clue in the diagnosis of autoimmune pancreatitis. Dig Dis Sci. 2011;56(5):1290-1294.
- Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013; 11(1):13–21.
- Engelbrecht MR, Katz SS, van Gulik TM, et al. Imaging of perihilar cholangiocarcinoma. AJR Am J Roentgenol. 2015; 204(4):782-791.
- Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract—an update. Cancer Imaging. 2014;22;14(1):14.
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657-1669.
- Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: What role? Radiology. 2010;257(1):24-39.
- Lim JH, Yoon KH, Kim SH, et al. Intraductal papillary mucinous tumor of the bile ducts. RadioGraphics. 2004; 24(1):53-67.
