Kawasaki Disease
By Porter KB, Towbin RB, Morgan D, Moore RA, Schaefer CM, Towbin AJ


Case Summary
An adolescent presented for follow up of aneurysms of the right coronary artery (RCA) and left anterior descending coronary artery (LAD). These were initially discovered when presenting as a baby with conjunctivitis, rash, skin desquamation, and diffuse erythema and swelling of the palms of the hands.
Imaging Findings
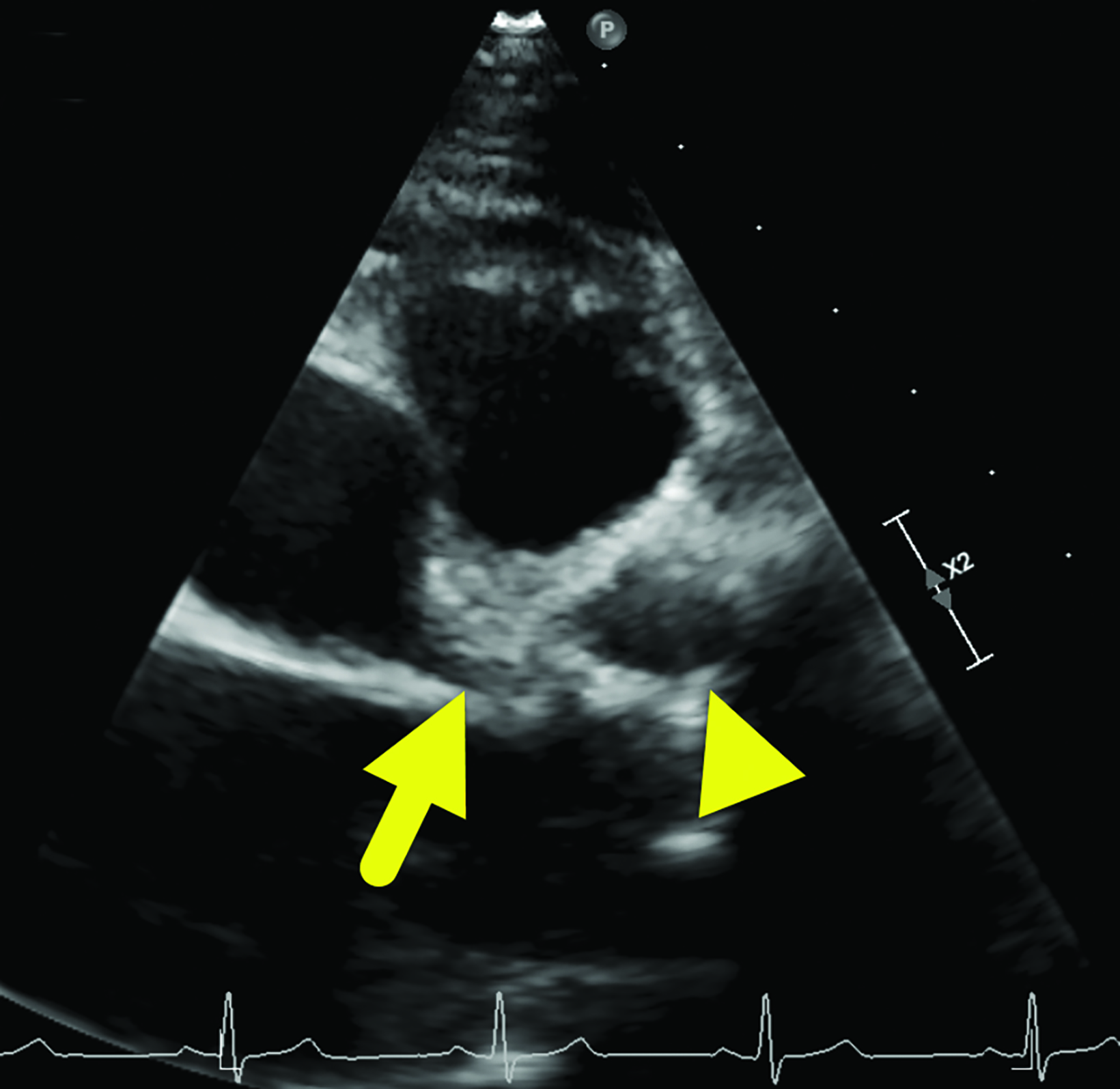
An echocardiogram (Figure 1) showed an aneurysm arising from the proximal LAD measuring 12 × 13.7 mm and another measuring 5.9 mm arising from the RCA.
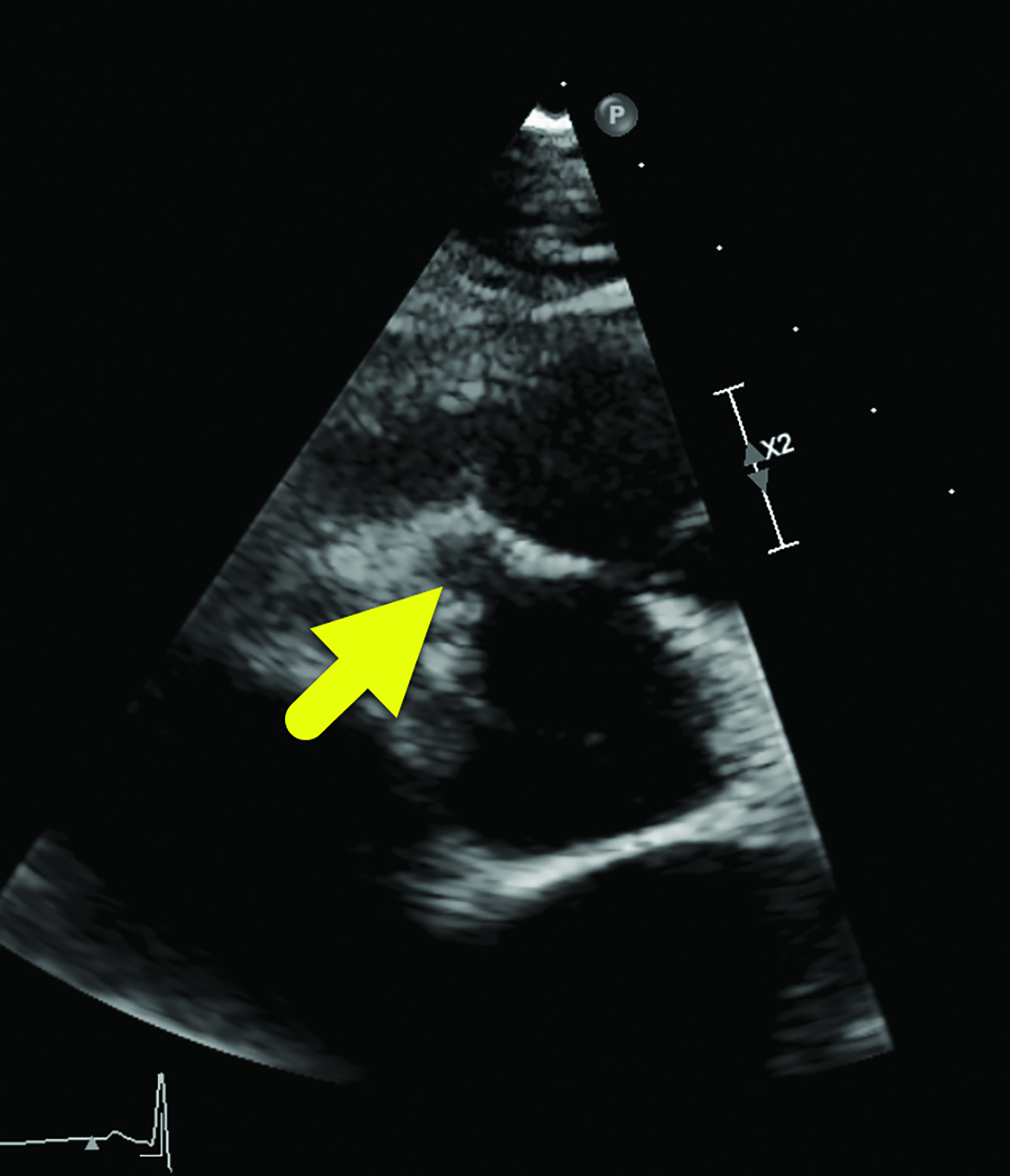
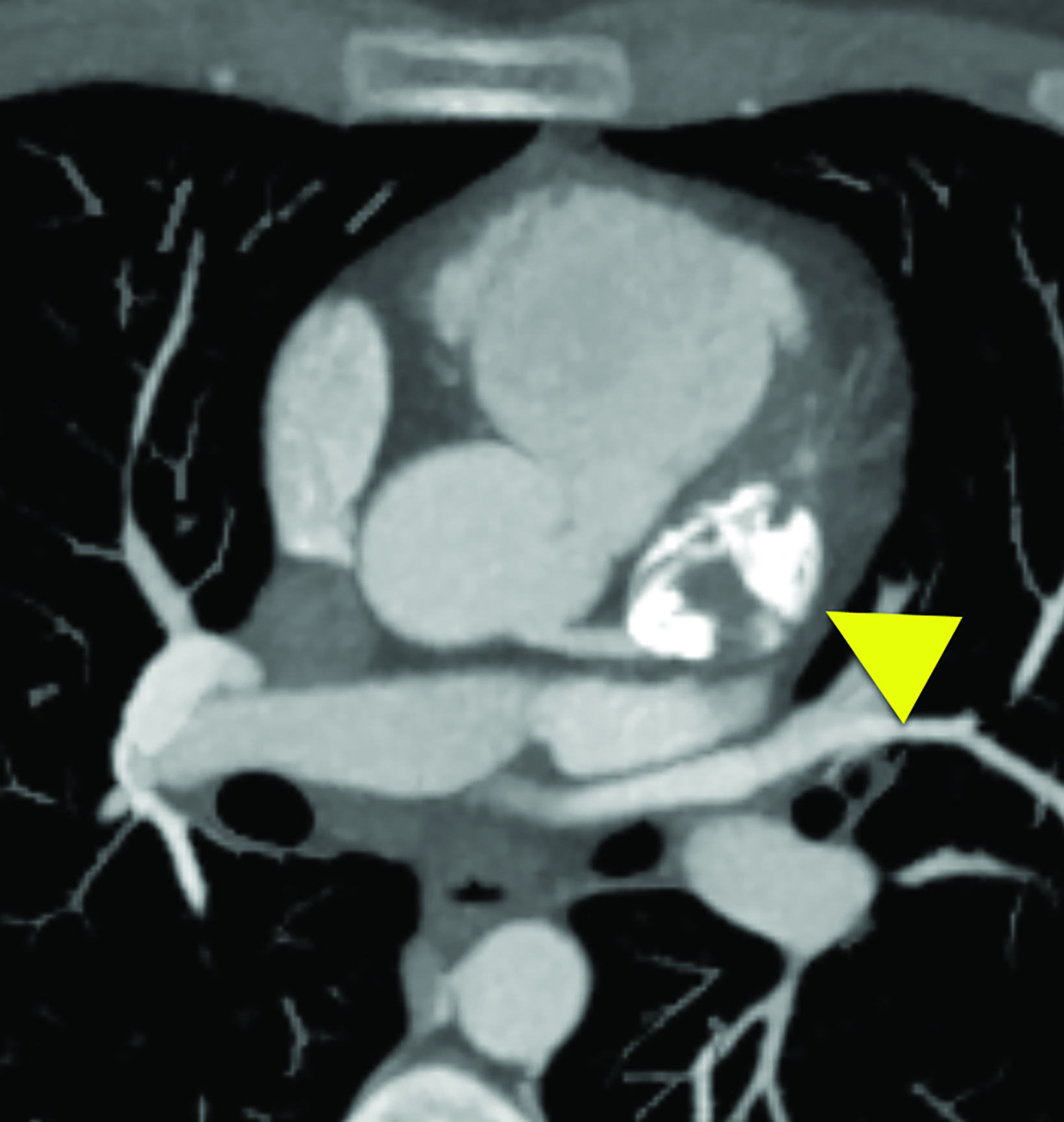
Cardiac MRI (Figure 2) showed interval enlargement of the LAD aneurysm, with mural thrombus.
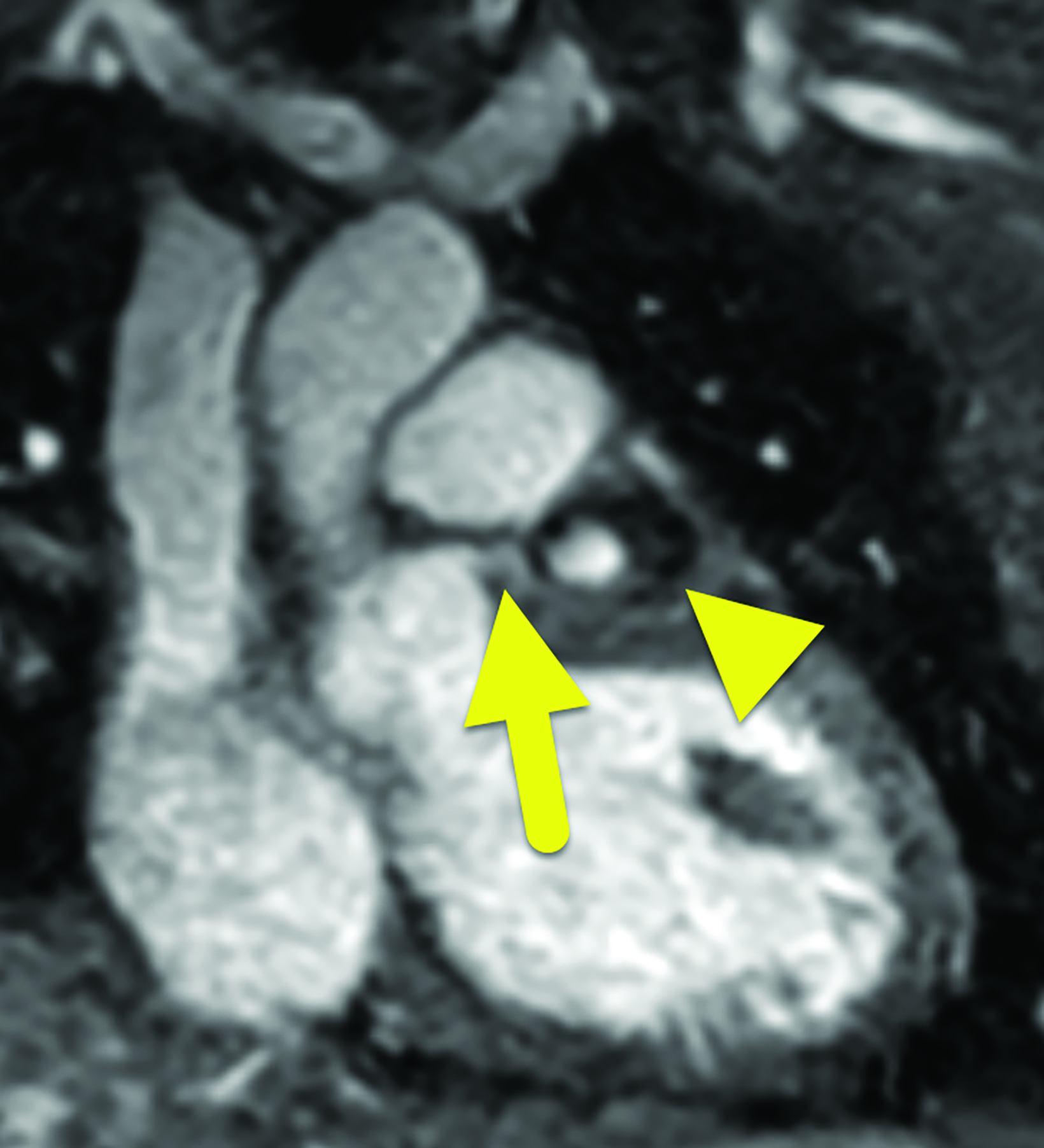
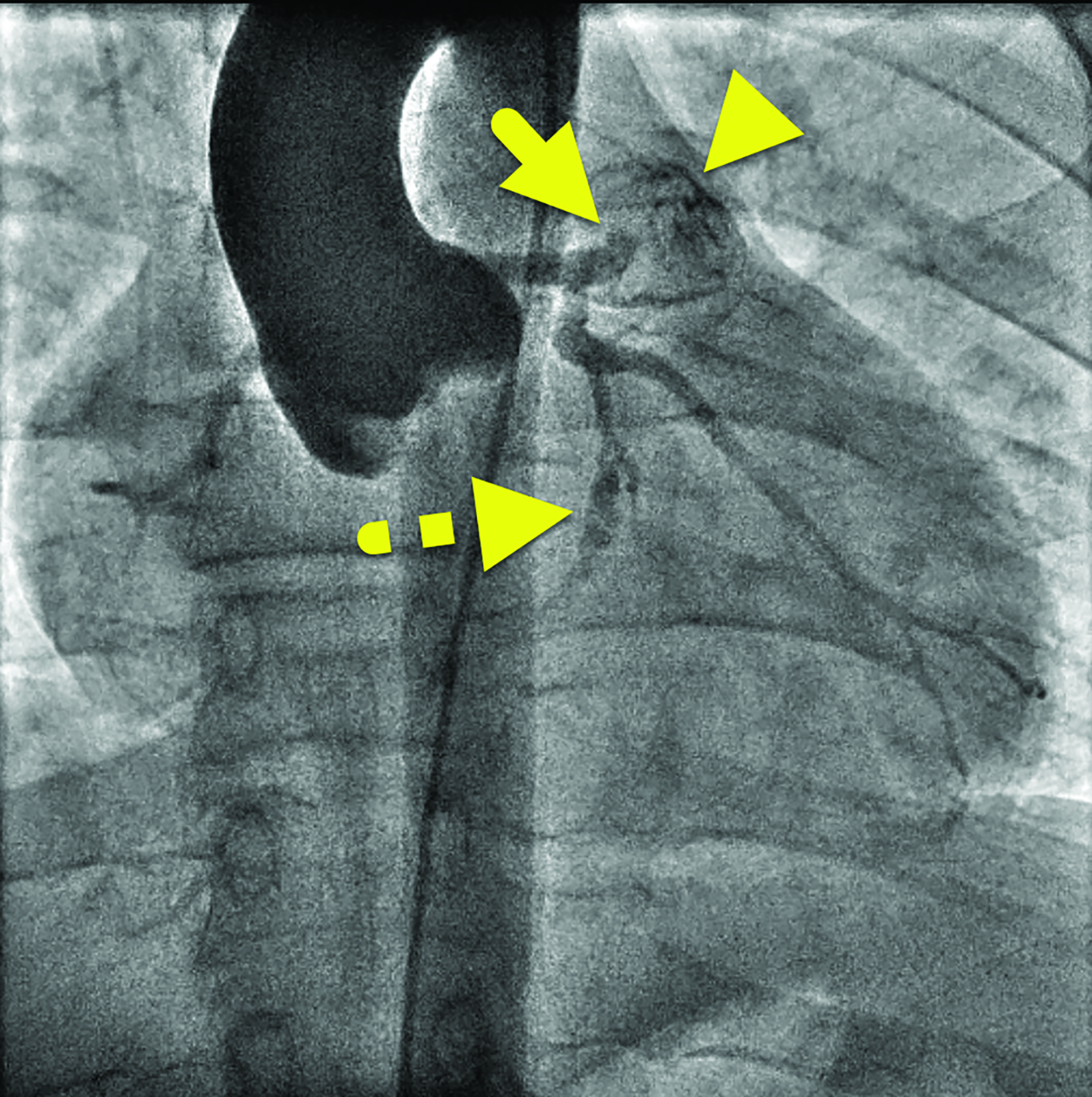
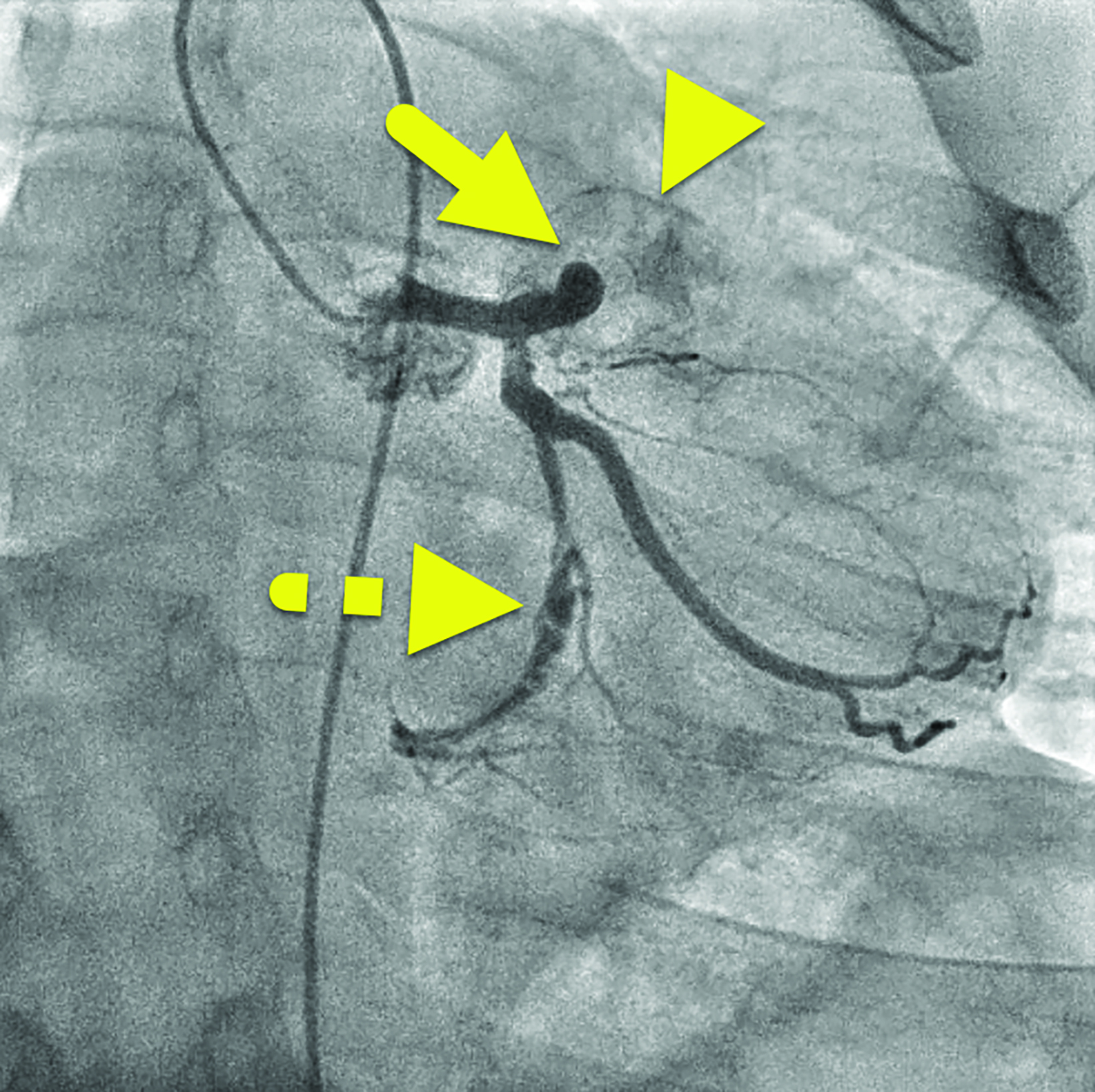
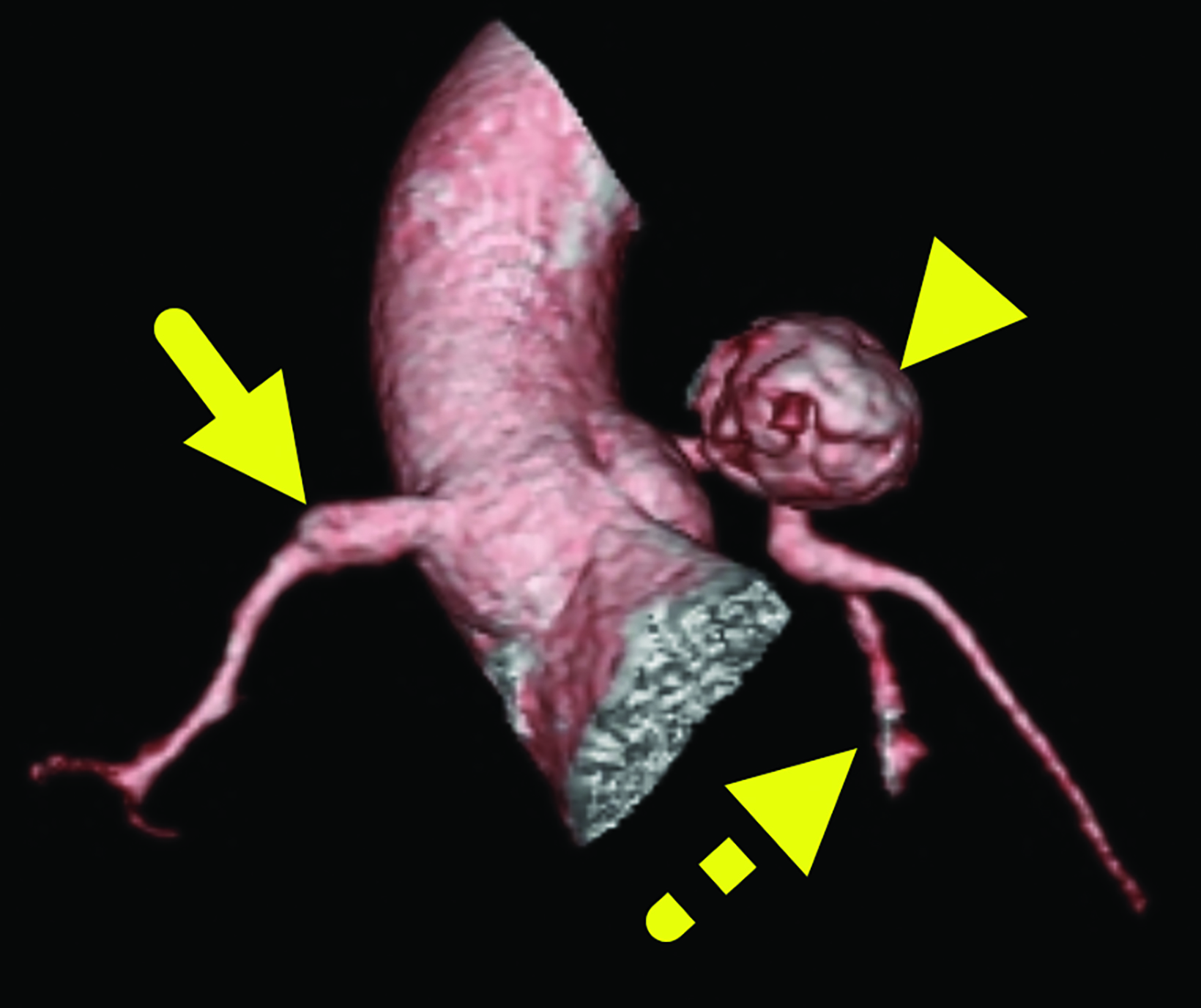
Cardiac catheterization (Figure 3) performed one year later showed no flow distal to the LAD aneurysm. Multiple collateral vessels were observed. Additionally, a new 9 mm aneurysm was present in the circumflex artery. The aneurysm arising from the RCA had enlarged considerably. The remainder of the vessel was tortuous, and a stenosis was suggested. The findings were confirmed on CT angiography (Figure 4).
Diagnosis
Kawasaki disease with coronary artery aneurysms.
Discussion
Kawasaki disease (KD) is an inflammatory condition that primarily affects small to medium-sized blood vessels in children under 5 years old, more common in boys. It is the leading cause of acquired heart conditions in children from developed nations.1 Although the cause of the disease is unknown, various theories center on its pathogenesis. These include an unknown, seasonal, airborne infectious agent or genetic abnormalities such as changes in caspase 3, inositol 1,4,5, trisphosphate kinase C, DC40, FCGR2a, and B cell lymphoid kinase that increase the risk of KD. The infectious and seasonal etiologies are supported primarily by clusters of epidemics that seem to have followed wind patterns occurring in Eastern Asia and several Western countries. The genetic theory is supported by variations associated with KD development, prognosis, and coronary artery aneurysm development.2
The epidemiology of KD is highly dependent on geographic location and ethnicity. The highest incidence of KD occurs in Japan, where 264 per 100,000 children are affected. This high incidence raises awareness and the associated mortality rate in Japan is less than 0.02%.2 In the continental United States, the incidence is notably lower, affecting 13-21 children per 100,000.2,3
Kawasaki disease is diagnosed on the basis of five clinical signs: 1) mucocutaneous changes such as erythema of the tongue (ie, “strawberry tongue”) and severe cheilitis (lip swelling and cracking); 2) nonexudative conjunctivitis with limbic sparing; 3) a polymorphous rash; 4) extremity changes such as edema and periungual desquamation; and 5) cervical lymphadenopathy.1,3,4
Patients must exhibit a fever for ≥ 5 days and at least four of the above signs for KD to be diagnosed. Other notable potential cardiac complications include myocarditis, valvular regurgitation, and coronary artery abnormalities. Incomplete (atypical) KD occurs when not all classic KD criteria are met; however, the children remain at risk for cardiovascular complications.5 Kawasaki disease is most often diagnosed when screening echocardiography reveals dilated coronary arteries. Patients with incomplete KD will often follow the same clinical course as classic KD.
Kawasaki disease generally presents in three phases: acute (1-2 weeks); subacute (2-4 weeks); and convalescent (4-8) weeks.4 The acute phase is associated with a high fever and the classic symptoms listed above. The subacute phase is usually asymptomatic.
During this phase, the patient is at high risk of developing associated cardiac complications such as coronary artery dilation and subsequent aneurysm formation. Approximately 25% of untreated and 5% of treated patients will develop coronary abnormalities.
The convalescent phase is also usually asymptomatic, but patients are now at a lower risk for developing cardiac symptoms owing to increased surveillance (including close clinical monitoring for fever recurrence) and a low threshold for treatment.
Laboratory findings, though nonspecific, can aid in making the diagnosis. Children can present with neutrophilia > 15,000 per mm;3 anemia; thrombocytosis > 450,000 per mm;3 and elevated ferritin, ALT, GGT, ESR, and C-reactive protein. Sterile pyuria of >10 WBCs per high powered field may also occur.5
During all phases of KD, imaging is used to detect potential cardiac anomalies. Echocardiography remains the imaging modality of choice.4 It is performed at the time of suspected KD diagnosis, up to 14 days later, and 6-8 weeks after that to survey for any cardiac abnormalities. If they are observed, imaging can be used for a longer duration to assess severity and prognosis.
Other potential studies that can be valuable are MR angiography, CT angiography, and cardiac catheterization. Cardiac catheterization may be warranted if coronary artery aneurysms are suspected.
Kawasaki disease is treated with intravenous immunoglobulin (IVIG) at a dose of 2 g/kg. Ideally, children should be treated within 10 days of the onset of fever. Effective IVIG therapy reduces the risk of a coronary artery lesion from 20-25% to 3-5%. However, up to 20% of patients will be termed “IVIG-resistant” and will experience recurrent fevers. A moderate dose (30-50 mg/kg/day) of acetyl- salicylic acid (ASA) is also given. In Japan and the United States, treatment with ASA is continued until the fever abates.6
Patients with coronary artery aneurysms are also treated with antithrombotic agents. The medication used is dependent on the size of the lesion. Patients with small aneurysms are continued on ASA for a period after the initial 6–8-week treatment regimen. Patients with aneurysms with an internal diameter >8 mm are generally started on anticoagulant therapy with warfarin or low molecular weight heparin.6
Conclusion
Kawasaki disease is an inflammatory vasculitis that primarily affects small to medium-sized vessels and is predominantly seen in male children under the age of 5 years. Those of Japanese ethnicity are at highest risk for the condition, which is associated with mucosal changes such as erythema and “strawberry tongue,” conjunctivitis, a polymorphous rash, extremity changes such as edema and periungual desquamation, and lymphadenopathy.
Coronary artery aneurysms are the feared sequelae of KD. Echocardiography is frequently used to assess for aneurysm development. The diseaes are generally treated with IVIG and ASA. Patients with aneurysms with an internal diameter greater than 8 mm are also treated with anticoagulant therapy.
References
- Singh, S., Jindal, A. K., & Pilania, R. K. (2018). Diagnosis of Kawasaki disease. Int J Rheum Dis., 21(1):36–44. doi:10.1111/1756-185X.13224. Epub. 2017 Nov 13. PMID: 29131549; PMCID: PMC7159575.
- Elakabawi K, Lin J, Jiao F, Guo N, Yuan Z. Kawasaki Disease: global burden and genetic background. Cardiol Res. 2020 Feb;11(1):9-14. doi: 10.14740/cr993. Epub 2020 Jan 26. PMID: 32095191; PMCID: PMC7011927.
- Harnden A, Takahashi M, Burgner D. Kawasaki disease. BMJ. 2009 May 5;338: b1514. doi: 10.1136/bmj. b1514. PMID: 19416993.
- Vervoort D, Donné M, Van Gysel D. Pitfalls in the diagnosis and management of Kawasaki disease: An update for the pediatric dermatologist. Pediatr Dermatol. 2018 Nov;35(6):743-747. doi: 10.1111/pde.13620. Epub 2018 Oct 18. PMID: 30338568.
- Rowley, A. H. (2015). The complexities of the diagnosis and management of Kawasaki disease. Infectious disease clinics of North America, 29(3), 525537. https://doi.org/10.1016/j.idc.2015.05.006
- Rife E, Gedalia A. Kawasaki disease: an update. Curr Rheumatol Rep. 2020 Sep 13;22(10):75. doi: 10.1007/s11926-020-00941-4. PMID: 32924089; PM-CID: PMC7487199.
