Konica Minolta Healthcare America receives FDA clearance for Dynamic Digital Radiography
 The US Food and Drug Administration (FDA) has given 510(k) clearance to Dynamic Digital Radiography (DDR), a technology that captures movement in a single exam developed by Konica Minolta Healthcare Americas. DDR produces medical images that depict movement and can be fully annotated, including diagrams, to help radiologists provide a more detailed clinical finding.
The US Food and Drug Administration (FDA) has given 510(k) clearance to Dynamic Digital Radiography (DDR), a technology that captures movement in a single exam developed by Konica Minolta Healthcare Americas. DDR produces medical images that depict movement and can be fully annotated, including diagrams, to help radiologists provide a more detailed clinical finding.
DDR enables clinicians to observe the dynamic interaction of anatomical structures, such as tissue and bone, with physiological changes over time. By bringing advanced cineradiography capabilities to digital radiography, and by providing quantifiable clinical information, DDR may increase the quality and specificity of diagnosis, and help clinicians rapidly answer clinical questions resulting in higher individualized care and reduced need for additional tests, according to Konica Minolta.
Konica Minolta said that two clinical areas where DDR can have an impact are in musculoskeletal (MSK) and thoracic imaging. DDR supports the diagnosis of MSK conditions by providing views of full patterns of articulatory mobility. With DDR, orthopedists and MSK specialists can acquire a full view of the MSK system in the supine and prone positions to view changes in the bone and articulations throughout the full range of motion. This information can be used to assess and monitor the spine and joints, such as the shoulder, knees, wrists and ankles, and enable the orthopedist or MSK specialist to provide a more detailed diagnosis quicker, reduce the need for additional imaging tests and enhance the quality of care.
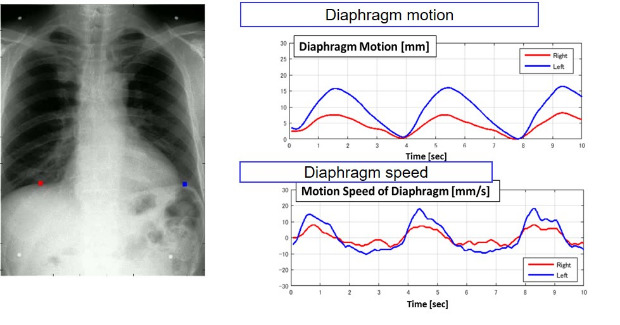
In thoracic and pulmonary imaging, DDR provides a full view of chest, lung and organ movement during the respiratory cycle. DDR also helps quantify movement, enabling the radiologist and pulmonologist to provide an enhanced assessment of pulmonary function to help determine the cause for dyspnea (shortness of breath). The potential causes for dyspnea are extensive and there are numerous tests used in diagnosis. In one imaging exam, DDR helps clinicians assess lung function, track lung movement to detect asymmetry (latent, paradoxical, limited or no movement), and differentiate asthma, obstruction, restriction or mixed conditions.
The company stated that DDR may overcome the limitations of pulmonary function tests, spirometers and static X-ray images that cannot identify differences between the left and right lung. With DDR’s advanced image processing and enhancement, physicians may track lesions that move throughout the respiratory cycle and identify blood vessel patterns and parenchymal abnormalities or pulmonary embolisms in many cases without a contrast agent. A bone suppression algorithm may help physicians differentiate calcifications from bone structures. Additional measurement tools can help identify and quantify differences in lung movement, which can be used to estimate lung volume; an edge tracking tool provides a graphical representation of lung movement throughout the respiratory cycle.
Konica Minolta said that DDR will be commercially released later in 2019. The company debuted the technology in Chicago at the 2018 RSNA annual meeting.
