Mimics of neoplasia: Common lesions and findings misdiagnosed as malignancy








Dr. Philips is an Assistant Professor, Dr. Restrepo is a Professor, and Dr. Chintapalli is a Professor in the Department of Radiology at the University of Texas Health Science Center, San Antonio, TX.
Several benign types of pathology can mimic the radiological appearance of neoplasm. An indeterminate or incorrect diagnosis may lead to unnecessary patient anxiety, biopsy, and even surgery. The recent surge of imaging utilization, particularly in computed tomography (CT), has contributed to an increase in detection of such lesions. A lack of pertinent patient history also contributes to greater difficulty in establishing a correct diagnosis.
In this review, the authors describe some of these benign entities that can mimic neoplasia on imaging and discuss the radiological features that may assist in reaching the correct diagnosis of these findings as benign conditions. For the purposes of this article, we have classified these benign etiologies into subgroups. Although any organization and distribution can be controversial, our classification aims to simplify the gamut of these lesions based on their predominant/distinctive etiology. Table 1 lists our classification scheme. Radiologists should be well acquainted with these pathologies and their unique imaging appearances.
Inflammatory pseudotumor
An inflammatory pseudotumor (IPT), also termed inflammatory myofibroblastic pseudotumor, is a rare lesion of unclear pathogenesis. It is characterized by reactive non-neoplastic proliferation of myofibroblastic spindle cells, accompanied by inflammatory cell infiltration within a myxoid stroma.1 IPT can occur in nearly every site in the body, from the central nervous system to the gastrointestinal tract (Figures 1 and 2). Some authors believe this is a low-grade fibrosarcoma with inflammatory cells. It is also thought to be the result of inflammation following minor trauma/surgery.2 An immune-autoimmune mechanism and an infectious cause have also been proposed.3 Organisms, such as the Epstein-Barr virus, have been found in splenic and nodal pseudotumors; actinomycetes and nocardiae are found in hepatic and pulmonary pseudotumors, respectively; and mycoplasma in pulmonary pseudotumors.4 Clinically, patients with inflammatory pseudotumor may have varying degrees of fever, thrombocytosis, and hypergammaglobulinemia
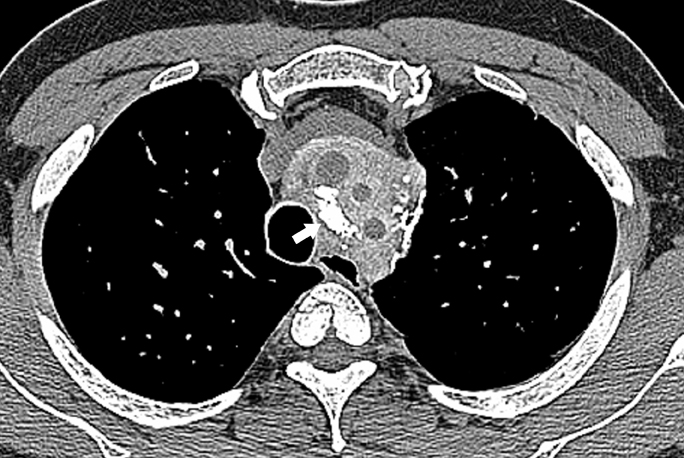
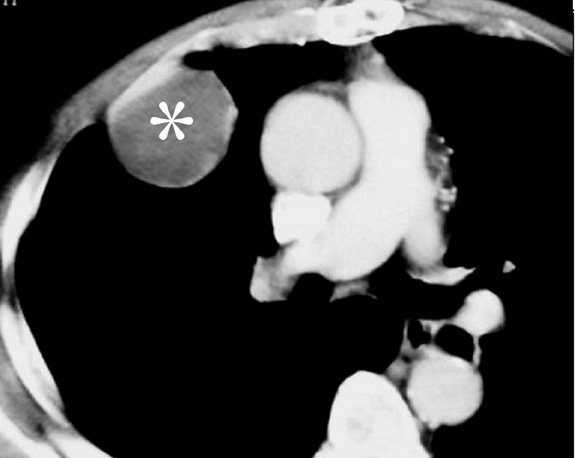
Pulmonary IPT is the most common primary lung mass seen in children and accounts for approximately 50% of benign intrapulmonary tumors seen in pediatric patients.5 Cough, fever, dyspnea, and hemoptysis are the usual presenting symptoms.6 On radiographs, it typically appears as a solitary, peripheral, sharply circumscribed, lobulated mass most commonly within the lower lobes.5 On CT scans, inflammatory pseudotumors have a variable and nonspecific appearance, but most commonly appear heterogeneous and show variable enhancement (Figure 3, click here to view this image in a DICOM viewer). Calcification within the lesion occurs more frequently in children.5,7
IPT of the liver has been recognized increasingly in children and young adults mainly in Asian countries.8 Most cases involve the right hepatic lobe and are solitary solid tumors. However, involvement of the porta hepatis or bile ducts, resulting in obstructive jaundice, can also be seen. Sclerosing cholangitis, phlebitis, and retroperitoneal fibrosis have been found in association with inflammatory pseudotumor of the liver.9 The IPT may spontaneously regress.8
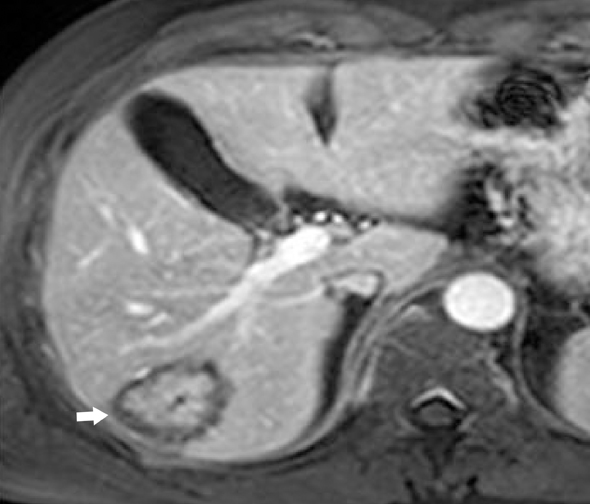
On sonography, IPT in the liver can be a hypo- as well as hyperechoic mass with transmission and visualization of multiple septa.10 On CT, they show soft-tissue attenuation with delayed and persistent enhancement due to fibrous content, although a variety of different enhancement patterns have been noted.8 On MR images, the mass is usually hypointense relative to skeletal muscle on T1-weighted images, hyperintense on T2-weighted images, and heterogeneously enhanced after administration of contrast material (Figure 4). Cholangiography may show biliary strictures of intra- or extrahepatic ducts.11 In addition, IPT may be associated with recurrent pyogenic cholangitis that leads to biliary stricture formation.
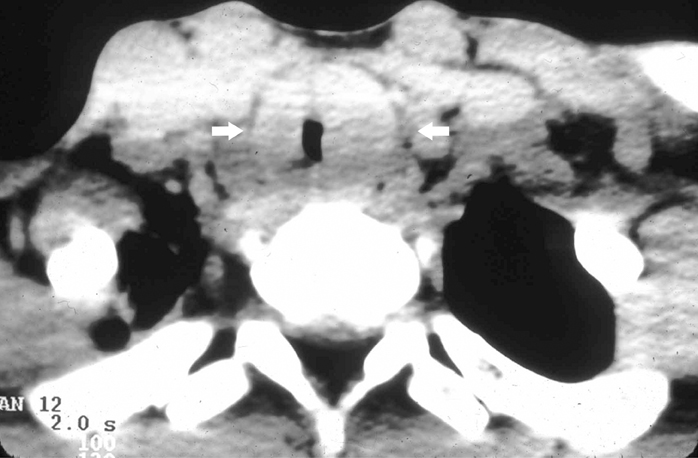
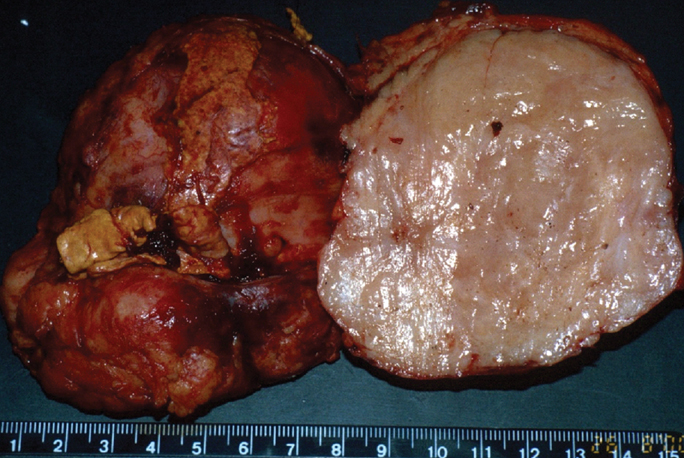
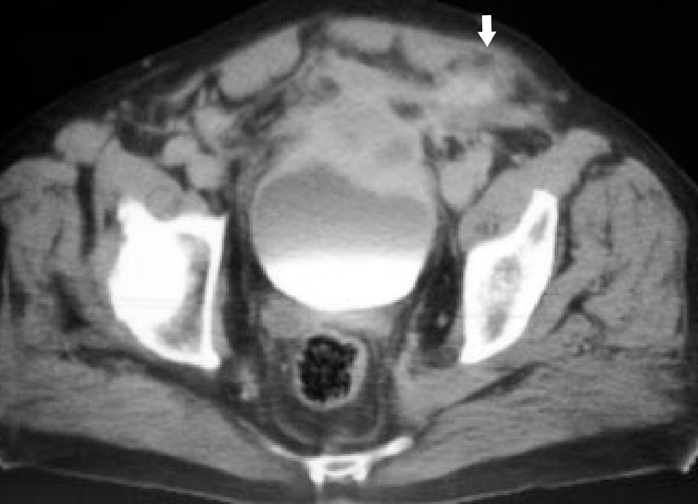
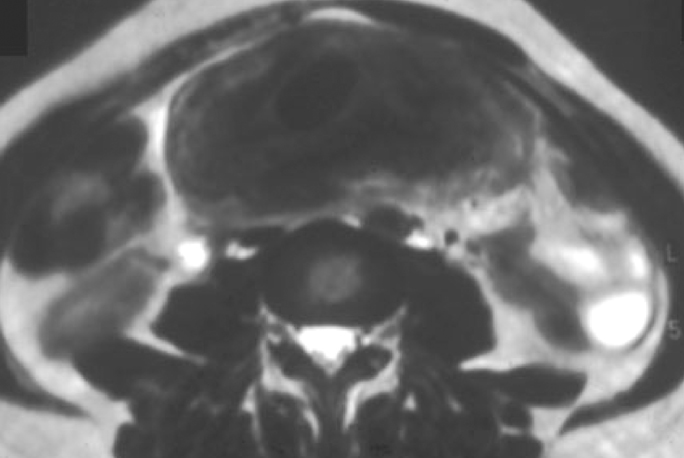
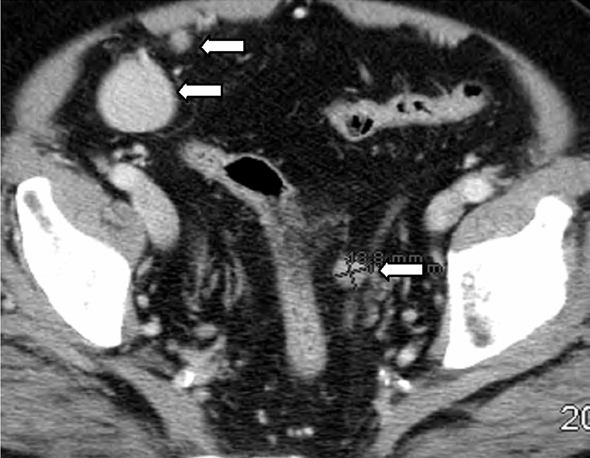
IPT of the urinary tract is extremely difficult to distinguish from malignant tumors clinically, radiologically, and histologically. Patients usually present with painless gross hematuria from exophytic and ulcerated lesions.12 Lesions within the urinary bladder are usually single, polypoid, intraluminal or submucosal masses with or without extension into the perivesical fat and they tend to spare the trigone area. (Figure 5).13 The maximum reported size has been 9 cm.14 Often IPT displays surface ulcerations and blood clots. IPT should be considered when an enhancing tumor is surrounded by a clot, particularly in young adults. Masses are usually hypervascular on Doppler imaging and show strong enhancement on contrast-enhanced CT or magnetic resonance imaging (MRI). Central necrosis may be observed within the lesion.15 Lesions may also show an infiltrating margin and can be accompanied by extensive sclerosis.14
Idiopathic fibrosing disorders/fibromatosis
The fibromatoses consist of a group of fibroproliferative conditions that are locally aggressive and have the capability to infiltrate or recur. This can occur in the mediastinum, small bowel mesentery, omentum, transverse or sigmoid mesocolon, and the retroperitoneum.16
Fibrosing mediastinitis
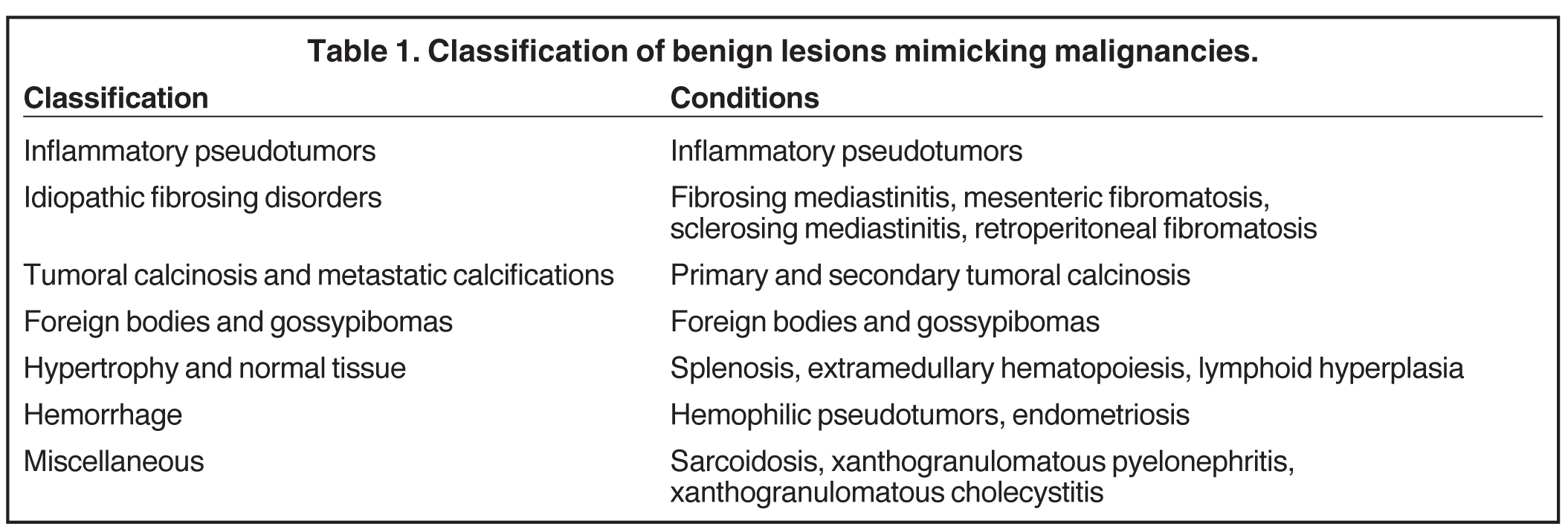
Fibrosing mediastinitis, also known as sclerosing mediastinitis, is characterized by proliferation of dense fibrous tissue within the mediastinum. Affected patients are typically young and present with signs and symptoms related to obstruction of vital mediastinal structures, such as central systemic veins, the esophagus, airways, and pulmonary arteries or veins. Two patterns of involvement have been described on CT scans: a focal and a diffuse pattern. The common focal form presents as a soft-tissue mass that is frequently calcified in the right paratracheal/subcarinal/hilar regions (Figure 6). The etiology of this form is postulated to be histoplasmosis in patients from the United States. In contrast, the diffuse form demonstrates an infiltrating, noncalcified mass affecting multiple compartments. This is often seen in association with other idiopathic fibrosing disorders, such as retroperitoneal fibrosis.
CT depicts the soft tissue mass and its extent. Intravenous contrast is useful for depiction encasement or obstruction of vessels with collaterals and also assessing for airway stenosis. Upper gastrointestinal series may also show narrowing of the esophagus when the patient presents with dysphagia (Figure 7). Two-dimensional or 3-dimensional reformation techniques may facilitate either surgical planning or local therapy of these lesions. On MRI, the T1-weighted magnetic resonance (MR) images show an intermediate signal mass with a variable signal on the T2-weighted MR.17
Mesenteric fibromatosis
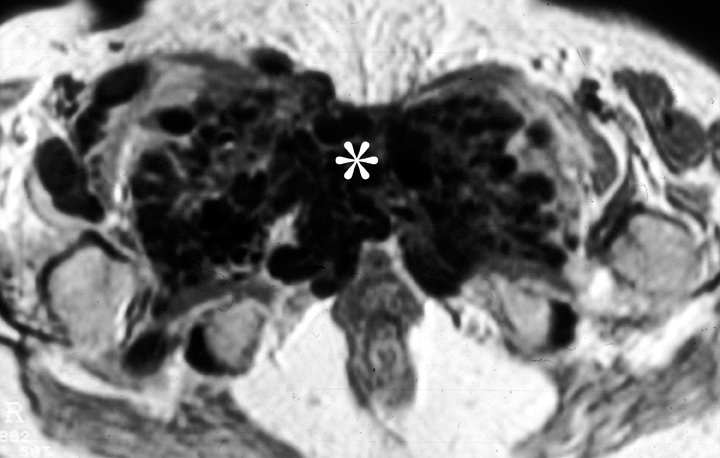
Mesenteric fibromatosis is a deep infiltrative fibroproliferative process that shows no gender or race predilection and occurs across a wide age range, 14-75 years.16 Thirteen percent of patients with mesenteric fibromatosis are found to have familial adenomatous polyposis (FAP), specifically, the Gardner variant.16 Prior abdominal surgery is an important risk factor for the development of mesenteric fibromatosis in patients with FAP. Complications of fibromatosis include bowel obstruction, fistulization, and even perforation. Mucosal ulceration may also occur as mesenteric fibromatosis may compromise the vasculature.18 The CT and MR imaging appearances are variable and related to its underlying histologic characteristics and vascularity.19 A homogeneous, soft-tissue attenuation is seen in tumors with a highly collagenous stroma, whereas a hypodense/intense appearance is common with a myxoid stroma (Figure 8). Contrast enhancement both on CT and MRI may vary from mild homogeneous to heterogeneous enhancement.20
Sclerosing mesenteritis
Sclerosing mesenteritis is an idiopathic disorder characterized by tumor-like masses in the mesentery that are composed of chronic, nonspecific inflammation, fat necrosis, and fibrosis. It usually involves the small bowel, but can also affect the colonic mesocolon.21 The average age at presentation is 60 years with increased frequency in males.22 On imaging, a soft-tissue mass is noted in the mesentery with retraction and shortening with kinking or fixation of the small bowel. Punctate or coarse calcifications can be present within the mass. The mass may envelop the mesenteric vessels and over time collateral vessels may develop. Preservation of fat around the mesenteric vessels is usually noted, referred to as the “fat ring sign.”23 This finding may help distinguish sclerosing mesenteritis from other mesenteric processes such as lymphoma, carcinoid tumor, or carcinomatosis.
Retroperitoneal fibrosis
Retroperitoneal fibrosis (RPF) often manifests as a paraspinal, well-demarcated but irregular retroperitoneal mass.24 The fibrosis first begins near the aorta and the iliac arteries, extending through the retroperitoneum to involve the ureters (Figure 9). The average age at presentation is 40-60 years, and men are 2 to 3 times more likely to develop retroperitoneal fibrosis than women.25 Avid enhancement on imaging may be seen in the acute stages. There may be little or no enhancement in the presence of advanced or chronic disease.26
RPF may be confused on imaging with a neoplastic process, such as lymphoma. It is important to note that RPF manifests as a plaque-like density whereas neoplastic infiltration results in peripheral nodularity and lobulation. In contrast to RPF, which tethers adjacent structures, lymphoma or other malignant processes usually display mass effect and displace the aorta and inferior vena cava anteriorly from the spine and push the ureters laterally.27 RPF almost always occurs caudal to the renal hilum.28
Tumoral calcinosis and metastatic calcifications
Primary tumoral calcinosis
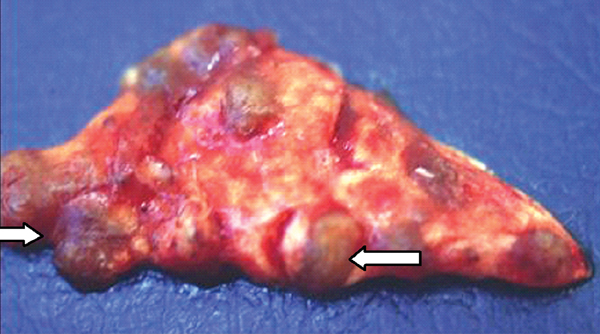
Primary tumoral calcinosis is a familial condition characterized by solitary or multiple painless, periarticular calcified masses. On imaging, tumoral calcinosis shows a rather typical appearance: amorphous, cystic, and multilobulated calcification located in a periarticular distribution. The greater trochanteric bursa is the most common site of involvement.29 Fluid-fluid levels caused by calcium layering may be seen within the lesion and are commonly termed the sedimentation sign.30 A homogeneous lesion suggests reduced metabolic activity and diminished ability to grow.31 A distinguishing finding of tumoral calcinosis is the absence of erosion or osseous destruction. T1-weighted MR images show low signal intensity, whereas the T2-weighted sequences generally show inhomogeneous high signal intensity even though there is a large amount of calcification.32
Secondary tumoral calcinosis
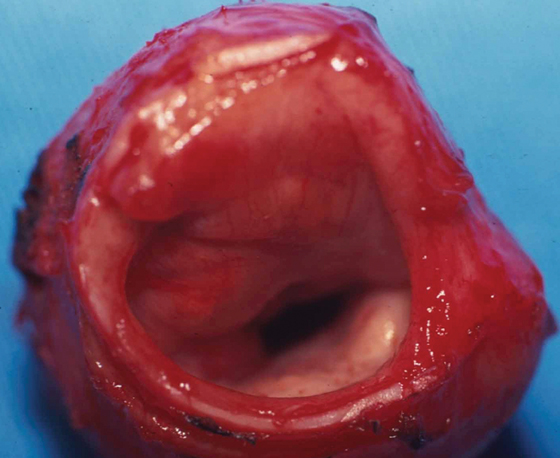
Chronic renal failure is the most common cause of a periarticular calcified mass, rather than the rarer primary tumoral calcinosis. Massive periarticular calcification has been attributed to hyperparathyroidism.33 Lesions of primary tumoral calcinosis and chronic renal failure show no radiologic or histologic differences (Figure 10).34 Hence, the differentiation is usually based on history, serum creatinine, and the glomerular filtration rate.
Foreign bodies and gossypibomas
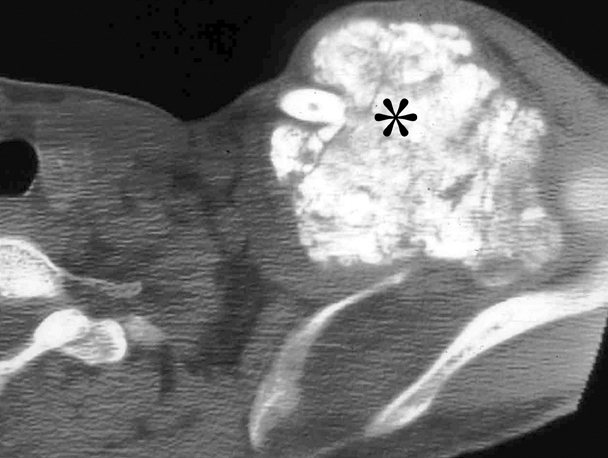
The term gossypiboma is derived from the Latin word gossypium, meaning cotton, and the Swahili word boma, meaning place of concealment.35 There are numerous case reports of retained foreign bodies in the literature, but the true incidence is thought to be under-reported secondary to possible legal ramifications of this oversight. A patient with a retained laparotomy sponge can present acutely with sepsis from abscess formation.36 Delayed presentations result from a fibrinous response, which is usually an aseptic process that creates adhesions and a thick capsule around the sponge. These masses are often confused with neoplasia.37 Most retained sponges are now impregnated with a radiopaque marker to facilitate radiographic detection. However, older sponges do not always have this marker (Figure 11). The retained sponge may also be a surgical towel that usually does not have any radio-opaque markers and often results in large gossypibomas.
On imaging, the most commonly described ultrasound appearance is that of an echogenic structure with sharply delineated posterior acoustic shadowing due to the reflective nature of the gauze fibers.38 CT usually shows a hypodense mass with a thick peripheral rim (Figure 12).39 A whorl-like or spongiform appearance is more typical, but may not always be seen.35 The internal structure of the gauze is best depicted on MRI as wavy low-signal intensity lines on T2-weighted images, which is a pathognomic finding.35
Hypertrophy and normal tissues
Splenosis
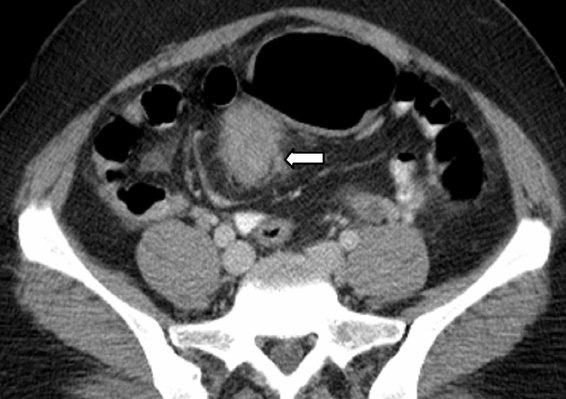
Splenosis is defined as the autotransplantation of splenic tissue resulting from the spillage of cells following splenic trauma or surgery.40 Splenic implants are generally multiple, have no characteristic shape, nor a hilum or a capsule. These can be located anywhere in the peritoneal cavity and also within the thorax following a diaphragmatic tear. On sonography, these implants have a homogeneously hypoechoic echotexture identical to the spleen.41 At CT, the attenuation is identical to that of normal spleen in all phases of the intravenous contrast material enhancement (Figure 13).42,43 The major differential diagnosis for splenosis includes adenopathy, peritoneal carcinomatosis, and lymphoma. If the diagnosis is in question after routine cross-sectional imaging, technetium99m tagged heat-damaged red blood cell scintigraphy or technetium99m sulfur colloid scintigraphy can be performed to confirm the diagnosis by showing uptake of radionuclide activity in ectopic splenic tissue.44
Extramedullary hematopiesis (EMH)
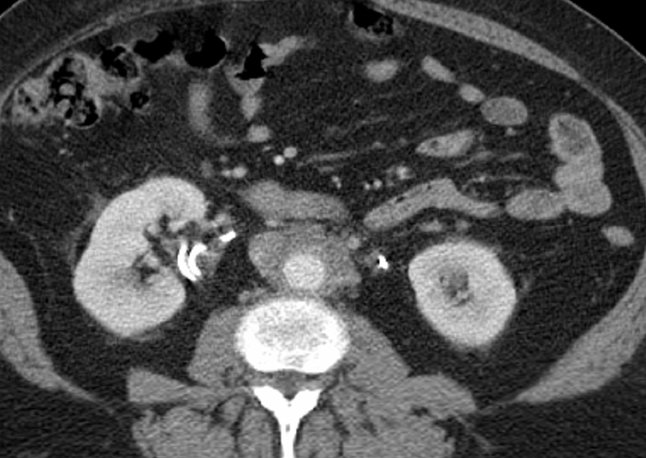
EMH is most commonly seen in the spleen and liver and occasionally in lymph nodes.1 Involvement of other organs, such as the pleura, lungs, gastrointestinal tract, breast, skin, brain, kidneys, and adrenal glands, has also been reported.45 The most common imaging manifestations are paraspinal masses and rib expansion seen frequently in β-thalassemia.46 The active hemopoietic masses are well-marginated and show mild-homogeneous enhancement on contrast-enhanced imaging. In contrast, old, burnt-out lesions may show iron deposition or fatty degeneration.46 EMH involving the pulmonary interstitium and mimicking an inflammatory or neoplastic diffuse interstitial process has been reported.47 In the abdomen, renal involvement is common, which may be parenchymal, intrapelvic, parapelvic, or perirenal. Perirenal masses typically present on imaging as uniform, enhancing perinephric masses that engulf the kidneys without distorting their shape (Figure 14). This appearance is often confused with bilateral renal lymphoma, and biopsy may be necessary to establish the diagnosis. Masses of hemopoietic elements can also involve the mesentery, presenting as nonspecific lesions that can be mistaken for lymphadenopathy or metastatic disease. Pelvic hemopoietic masses with a predilection for the presacral region are rare; however, these should be included in the differential diagnosis along with other presacral lesions, including chordoma. Identifying EMH is important as treatment with steroids and external beam radiation is effective and usually long-lasting.48
Lymphomatoid hyperplasia
Castleman disease, also known as angiofollicular or benign giant lymph node hyperplasia, is an uncommon lymphoproliferative disorder.49 Castleman disease involves the thorax in 70% of cases; the abdomen in 10% to 15%, retroperitoneum and pelvis in 10% to 15% and the neck on occasion. Castleman disease can present as a unicentric form or a multicentric form. The unicentric form (90% of cases) demonstrates hyaline-vascular histology and is amenable to surgical treatment. The multicentric form typically occurs in an elderly population and shows plasma cell histology and is usually associated with more complicated systemic manifestations.50
Thoracic Castleman disease usually occurs in the mediastinum and hilum and manifests as a rounded solitary mediastinal or hilar mass in asymptomatic patients (Figure 15).49 The mediastinal form may mimic thymoma, lymphoma, or neurogenic tumors.49 On the basis of CT, 3 types of imaging appearances are noted, including a well-defined solitary lesion (50% of cases); mass with involvement/invasion of contiguous structures (40% of cases); or confluent lymphadenopathy in a single mediastinal compartment (10% of cases).50 Homogeneously intense contrast enhancement, reflecting hypervascularity of the lesion, is considered to be a characteristic CT finding. On CT, 5% to 10% of Castleman disease showed intralesional calcifications, typically being discrete in morphology.50,51 Intrathoracic multicentric Castleman disease typically exhibits bilateral hilar and mediastinal lymphadenopathy; centrilobular nodular opacities (Figure16). Less common imaging appearances include ground-glass opacities, consolidation and bronchiectasis.51 In the abdomen, multicentric Castleman disease is characterized by diffuse lymphadenopathy, hepatomegaly, splenomegaly, ascites, and thickening of the retroperitoneal fascia. The major differential diagnosis is lymphoma.
Hemorrhage
Hemophilic pseudotumors
A hemophilic pseudotumor is an encapsulated, chronic, slowly growing hematoma. This is a rare complication of hemophilia, occurring in 1% to 2% of persons with severe disease.52 Pseudotumors usually occur in soft tissues (often intramuscular), but occasionally may originate in the bone or in a subperiosteal location. Pseudotumors that occur in muscles can progress to cause severe pressure erosion of adjacent bone.53 The bones most commonly involved are the femur, pelvis, tibia, and bones of the hand. Compression of nerves or pathologic fractures may produce pain or neurologic deficits.54
Pseudotumors have a variable radiographic appearance, however, are usually lytic with a well-defined margin. Lesions may be intramedullary or eccentric in location and are often expansile. CT and MR images are useful for determining the extent of the pseudotumor and for defining surrounding neurovascular structures and joints (Figure 17).55 On MRI, there are heterogeneous low- and high-signal-intensity areas internally on both T1- and T2-weighted images, findings that represent blood products in various stages of evolution.53
Many bone tumors as well as infectious processes, such as echinococcosis, may resemble pseudotumors on imaging since many of these lesions show internal hemorrhage. Percutaneous drainage or biopsy is contraindicated due to the high prevalence of complications, including life-threatening bleeding, fistula formation, and infection.54 Therefore, it is vital that the radiologist make the diagnosis of pseudotumor based on patient history and imaging findings.
Endometriosis
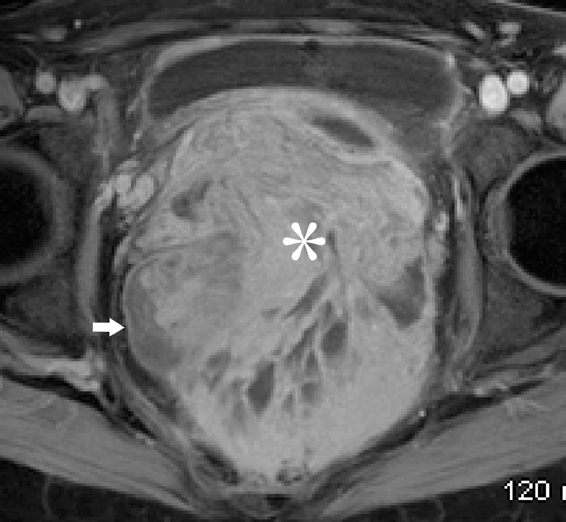
Endometriosis is defined as the presence of endometrial glands and/or stroma in locations outside the uterus. The prevalence has been quoted to range around 5% to 10 % in both symptomatic and asymptomatic women.56 The common sites of disease include the ovaries, uterine ligaments, serosal surfaces, cul-de-sac, fallopian tubes, rectosigmoid, and urinary bladder.57 Gastrointestinal involvement is estimated to occur in 12% to 37% of patients.58 Areas frequently involved are the rectosigmoid colon, appendix, cecum, and distal ileum. Implants are usually serosal, but can erode through the subserosal layers, with thickening and fibrosis of the muscularis propria (Figure 18). Invasive endometriosis is typically found in the rectovaginal septum and fibromuscular pelvic structures, such as the uterine ligaments and the muscular walls of pelvic organs accompanied by smooth muscle proliferation and fibrotic reaction. Solid masses in the pelvis may simulate metastatic peritoneal implants (Figure 18).
Pleuropulmonary endometriosis is usually coexistent with pelvic endometriosis and usually occurs 5 years following the diagnosis of pelvic endometriosis. The imaging findings include pneumothorax, hemothorax, and lung nodules. The pleural lesions are almost exclusively right-sided, whereas lung lesions do not show any such predilection (Figure 18).59
Miscellaneous conditions
Sarcoidosis
Sarcoidosis is a systemic disorder characterized by noncaseating granulomas with proliferation of epithelioid cells. Young and middle-aged patients, especially women, are most commonly affected.60 Ninety percent of patients with sarcoidosis show pulmonary involvement, which commonly manifests as asymptomatic mediastinal adenopathy.60
Less commonly seen findings of pulmonary sarcoidosis include multiple miliary nodules, bronchial-wall thickening, and ground-glass attenuation. The latter may reflect the presence of microscopic interstitial granulomas.
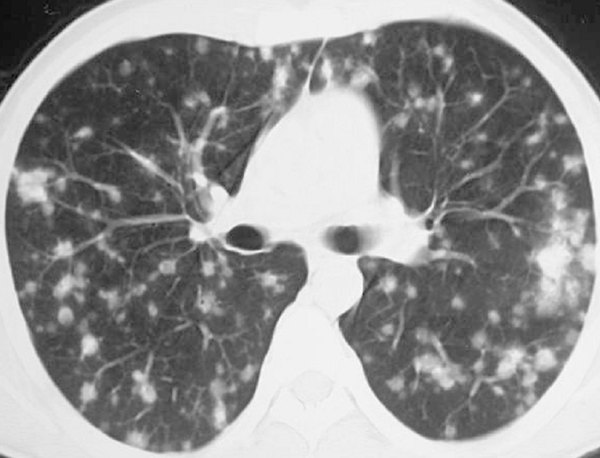
Sarcoidosis may also involve the abdomen, especially the liver and spleen; however, the imaging findings usually include only hepatosplenomegaly. Rarely coalescing granulomas become apparent as multiple hypointense or hypoattenuating nodules.61 Splenic nodules are larger and more common than hepatic lesions.61 Twenty-five percent of patients have normal findings at chest radiography. Multiple nodules in hepatic sarcoidosis are easily mistaken for more common diseases, including metastases and lymphoma. When there is no other clinical evidence of sarcoidosis, liver biopsy may be considered. Rarely, renal sarcoidosis manifests as multiple low-attenuation tumor-like nodules that can mimic lymphoma or metastatic tumors (Figure 19).62,63
Xanthogranulomatous pyelonephritis
Xanthogranulomatous pyelonephritis is an uncommon form of chronic renal parenchymal infection.64 The renal parenchyma is usually diffusely involved; however, focal involvement is also noted. The kidney is diffusely enlarged with replacement of the renal parenchyma by multiple low-attenuation rounded masses with attenuation values of 10-15 HU. A “staghorn” calculus is commonly associated.64 Extension to involve the peri- and paranenal spaces, ipsilateral psoas muscle, diaphragm, posterior abdominal wall, skin, and bowel may be present and mimic lymphoma or renal cancer.
Conclusion
The increasing use of CT and MRI to establish or exclude a wide variety of diagnoses in many different clinical settings will likely increase recognition of non-neoplastic lesions that can simulate malignancies. Some of these lesions are indeed benign and self-limited, while others can be locally aggressive and require specific treatment. It is important to recognize that many of these lesions have unique imaging appearances that can aid in distinguishing them from malignancy. Signs and symptoms have a major role in narrowing diagnostic considerations. Hopefully, this article, while not comprehensive, will help guide radiologists in distinguishing these conditions from true malignancies.
References
- Scott L, Blair G, Taylor G, et al. Inflammatory pseudotumors in children. J Pediatr Surg. 1988;23:755-758.
- Sanders BM, West KW, Gingalewski C, et al. Inflammatory pseudotumor of the alimentary tract: Clinical and surgical experience. J Pediatr Surg. 2001;36:169-173.
- Stark P, Sandbank JC, Rudnicki C, Zahavi I. Inflammatory pseudotumor of the heart with vasculitis and venous thrombosis. Chest. 1992;102:1884-1885.
- Dehner LP. The enigmatic inflammatory pseudotumours: The current state of our understanding, or misunderstanding. J Pathol. 2000;192:277-279.
- Agrons GA, Rosado-de-Christenson ML, Kirejczyk WM, et al. Pulmonary inflammatory pseudotumor: Radiologic features. Radiology. 1998;206:511-518.
- Cohen MC, Kaschula RO. Primary pulmonary tumors in childhood: A review of 31 years’ experience and the literature. Pediatr Pulmonol. 1992;14:222-232.
- Patankar T, Prasad S, Shenoy A, Rathod K. Pulmonary inflammatory pseudotumour in children. Australas Radiol. 2000;44:318-320.
- Levy S, Sauvanet A, Diebold MD, et al. Spontaneous regression
of an inflammatory pseudotumor of the liver presenting as an obstructing
malignant biliary tumor. Gastrointest Endosc. 2001;53:
371-374. - Torzilli G, Inoue K, Midorikawa Y, et al. Inflammatory pseudotumors of the liver: Prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001;48:1118-1123.
- Nam KJ, Kang HK, Lim JH. Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR Am J Roentgenol. 1996;167:485-487.
- Tublin ME, Moser AJ, Marsh JW, Gamblin TC. Biliary inflammatory pseudotumor: Imaging features in seven patients. AJR Am J Roentgenol. 2007;188:W44-48.
- Gugliada K, Nardi PM, Borenstein MS, Torno RB. Inflammatory pseudosarcoma (pseudotumor) of the bladder. Radiology. 1991;179:66-68.
- Fujiwara T, Sugimura K, Imaoka I, Igawa M. Inflammatory pseudotumor of the bladder: MR findings. J Comput Assist Tomogr. 1999;23:
558-561. - Ricchiuti DJ, Ricchiuti VS, Ricchiuti RR, Qadri AM, Resnick MI. Fibrous inflammatory pseudotumor of the bladder. Rev Urol. 2000;2:232-235.
- Sugita R, Saito M, Miura M, Yuda F. Inflammatory pseudotumour of the bladder: CT and MRI findings. Br J Radiol. 1999;72:809-811.
- Burke AP, Sobin LH, Shekitka KM, et al. Intra-abdominal fibromatosis. A pathologic analysis of 130 tumors with comparison of clinical subgroups. Am J Surg Pathol. 1990;14:335-441.
- Rholl KS, Levitt RG, Glazer HS. Magnetic resonance imaging of fibrosing mediastinitis. AJR Am J Roentgenol. 1985;145:255-259.
- Church JM. Mucosal ischemia caused by desmoid tumors in patients with familial adenomatous polyposis: Report of four cases. Dis Colon Rectum. 1998;41:661-663.
- Casillas J, Sais GJ, Greve JL, et al. Imaging of intra- and extra-abdominal desmoid tumors. Radiographics. 1991;11:959-968.
- Levy AD, Rimola J, Mehrotra AK, Sobin LH. From the archives of the AFIP: Benign fibrous tumors and tumor like lesions of the mesentery: Radiologic-pathologic correlation. Radiographics. 2006;26:245-264.
- Han SY, Koehler RE, Keller FS, et al. Retractile mesenteritis involving the colon: Pathologic and radiologic correlation (case report). AJR Am J Roentgenol. 1986;147:268-270.
- Emory TS, Monihan JM, Carr NJ, Sobin LH. Sclerosing mesenteritis, mesenteric panniculitis and mesenteric lipodystrophy: A single entity? Am J Surg Pathol. 1997;21:392-398.
- Valls C. Fat-ring sign in sclerosing mesenteritis. AJR Am J Roentgenol. 2000;174:259-260.
- Kottra JJ, Dunnick NR. Retroperitoneal fibrosis. Radiol Clin North Am. 1996;34:1259-1275.
- Lepor H, Walsh PC. Idiopathic retroperitoneal fibrosis. J Urol. 1979;122:1-6.
- Feinstein RS, Gatewood OM, Goldman SM, et al. Computerized tomography in the diagnosis of retroperitoneal fibrosis. J Urol. 1981;126:255-259.
- Amis ES, Jr. Retroperitoneal fibrosis. AJR Am J Roentgenol. 1991;157:321-329.
- Brun B, Laursen K, Sorensen IN, et al. CT in retroperitoneal fibrosis. AJR Am J Roentgenol. 1981;137:535-358.
- Palmer PE. Tumoural calcinosis. Br J Radiol. 1966;39:518-525.
- Hug I, Guncaga J. Tumoral calcinosis with sedimentation sign. Br J Radiol. 1974;47:734-736.
- Harkess JW, Peters HJ. Tumoral calcinosis. A report of six cases. J Bone Joint Surg Am. 967;49:721-731.
- Martinez S, Vogler JB, 3rd, Harrelson JM, Lyles KW. Imaging of tumoral calcinosis: New observations. Radiology. 1990;174:215-522.
- Eisenberg B, Tzamaloukas AH, Hartshorne MF, et al. Periarticular tumoral calcinosis and hypercalcemia in a hemodialysis patient without hyperparathyroidism: A case report. J Nucl Med. 1990;31:1099-1103.
- Gordon LF, Arger PH, Dalinka MK, Coleman BG. Computed tomography in soft tissue calcification
layering. J Comput Assist Tomogr. 1984;8:71-73. - O’Connor AR, Coakley FV, Meng MV, Eberhardt SC. Imaging of retained surgical sponges in the abdomen and pelvis. AJR Am J Roentgenol. 2003;180:481-489.
- Apter S, Hertz M, Rubinstein ZJ, Zissin R. Gossypiboma in the early post-operative period: A diagnostic problem. Clin Radiol. 1990;42:128-129.
- Sturdy JH, Baird RM, Gerein AN. Surgical sponges: A cause of granuloma and adhesion formation. Ann Surg. 1967;165:128-134.
- Yamato M, Ido K, Izutsu M, et al. CT and ultrasound findings of surgically retained sponges and towels. J Comput Assist Tomogr. 1987;11:1003-1006.
- Bani-Hani KE, Gharaibeh KA, Yaghan RJ. Retained surgical sponges (gossypiboma). Asian J Surg. 2005;28:109-115.
- Fleming CR, Dickson ER, Harrison EG, Jr. Splenosis: Autotransplantation of splenic tissue. Am J Med. 1976;61:414-419.
- Maillard JC, Menu Y, Scherrer A, et al. Intraperitoneal splenosis: Diagnosis by ultrasound and computed tomography. Gastrointest Radiol. 1989;14:179-180.
- Mendelson DS, Cohen BA, Armas RR. CT appearance of splenosis. J Comput Assist Tomogr. 1982;6:1188-1190.
- Lin WC, Lee RC, Chiang JH, et al. MR features of abdominal splenosis. AJR Am J Roentgenol. 2003;180:493-496.
- Alvarez R, Diehl KM, Avram A, et al. Localization of splenosis using 99mTc-damaged red blood cell SPECT/CT and intraoperative gamma probe measurements. Eur J Nucl Med Mol Imaging. 2007;34:969.
- Bronn LJ, Paquelet JR, Tetalman MR. Intrathoracic extramedullary hematopoiesis: Appearance on 99mTc sulfur colloid marrow scan. AJR Am J Roentgenol. 1980;134:1254-5125.
- Tsitouridis J, Stamos S, Hassapopoulou E, et al. Extramedullary paraspinal hematopoiesis in thalassemia: CT and MRI evaluation. Eur J Radiol. 1999;30:33-38.
- Coates GG, Eisenberg B, Dail DH. Tc-99m sulfur colloid demonstration of diffuse pulmonary interstitial extramedullary hematopoiesis in a patient with myelofibrosis. A case report and review of the literature. Clin Nucl Med. 1994;19:1079-1084.
- Munn RK, Kramer CA, Arnold SM. Spinal cord compression due to extramedullary hematopoiesis in beta-thalassemia intermedia. Int J Radiat Oncol Biol Phys. 1998;42:607-609.
- Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29:670-683.
- McAdams HP, Rosado-de-Christenson M, Fishback NF, Templeton PA. Castleman disease of the thorax: Radiologic features with clinical and histopathologic correlation. Radiology. 1998;209:221-228.
- Johkoh T, Muller NL, Ichikado K, et al. Intrathoracic multicentric Castleman disease: CT findings in 12 patients. Radiology. 1998;209:477-481.
- Ahlberg AK. On the natural history of hemophilic pseudotumor. J Bone Joint Surg Am. 1975; 57:1133-1136.
- Jaovisidha S, Ryu KN, Hodler J, et al. Hemophilic pseudotumor: Spectrum of MR findings. Skeletal Radiol. 1997;26:468-474.
- Magallon M, Monteagudo J, Altisent C, et al. Hemophilic pseudotumor: Multicenter experience over a 25-year period. Am J Hematol. 1994;45:103-108.
- Hermann G, Gilbert MS, Abdelwahab IF. Hemophilia: Evaluation of musculoskeletal involvement with CT, sonography, and MR imaging. AJR Am J Roentgenol. 1992;158:119-123.
- Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759-1769.
- Ascher SM, Agrawal R, Bis KG, et al. Endometriosis: Appearance and detection with conventional and contrast-enhanced fat-suppressed spin-echo techniques. J Magn Reson Imaging. 1995;5:251-257.
- Blaustein A, Kurman R. Blaustein’s Pathology of the female genital tract. 4th ed. New York, NY: Springer-Verlag; 1994.
- Clement, PB. Diseases of the peritoneum. In: Blaustein A, Kurman R, eds. Blaustein’s Pathology of the female genital tract. 4th ed. New York, NY: Springer-Verlag; 1994.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Warshauer DM, Molina PL, Hamman SM, et al. Nodular sarcoidosis of the liver and spleen: Analysis of 32 cases. Radiology. 1995;195:757-762.
- Sato A. Renal dysfunction in patients with sarcoidosis. Intern Med. 1996;35:523-524.
- Hughes JJ, Wilder WM. Computed tomography of renal sarcoidosis. J Comput Assist Tomogr. 1988;12:1057-1058.
- Malek RS, Greene LF, DeWeerd JH, Farrow GM. Xanthogranulomatous pyelonephritis. Br J Urol. 1972;44:296-308.
