MRI of right lower quadrant pain in pregnancy: Appendicitis and mimickers
Images









Acute right lower quadrant (RLQ) pain in pregnancy presents a unique diagnostic challenge, encompassing a broad differential of gastrointestinal, gynecological, obstetrical, and renal causes. The possibility of acute appendicitis must be specifically ruled out as it is the most common cause of surgical intervention in pregnancy and requires urgent management.1-3 Acute appendicitis has an estimated incidence of 1/500 to 1/1500 pregnancies, with approximately 50% of all cases occurring in the second trimester.1,2 Challenges in clinical assessment, diagnostic imaging and greater operative risk in the pregnant patient contribute to a 15-40% incidence of appendiceal perforation and 8% risk of fetal loss from acute appendicitis during pregnancy.2, 3
The classic clinical presentation of acute appendicitis, including RLQ pain, nausea, vomiting, fever, and leukocytosis, is confounded by normal changes in maternal physiology during pregnancy. Clinical diagnosis is made difficult by abdominal pain and changing location of the appendix from an enlarging uterus, nausea and vomiting from morning sickness or hyperemesis gravidarum, and physiological fever and leukocytosis.4 Pregnant patients also frequently present atypically and have equivocal clinical findings necessitating medical imaging for diagnosis.
Diagnostic imaging in pregnancy is complicated by risk of fetal teratogenicity from ionizing radiation from computed tomography (CT) imaging. Magnetic resonance (MR) imaging produces no ionizing radiation and offers excellent spatial and soft-tissue resolution of abdominal structures. While its use is limited by higher cost, lower availability, and longer acquisition time, technological advances in fast imaging sequences and reduction of motion artifact have made MR imaging preferred over CT in pregnant patients. The American College of Radiology currently recommends graded compression ultrasound as the first-line investigation for suspected appendicitis in pregnancy, with MR imaging as the second-line investigation if ultrasound results are nondiagnostic.5
In this article, we present our institution’s MR imaging protocol for evaluation of suspected appendicitis in the pregnant patient and illustrate the MR imaging appearance of both the normal appendix and spectrum of appearances of acute appendicitis seen in pregnancy. Lastly, we discuss the key MR imaging findings of common genitourinary and gastrointestinal differential etiologies of RLQ pain occurring in pregnancy.
MR Imaging technique
The use of oral and intravenous contrast agents in pregnant patients remains controversial. Bowel preparation with oral contrast agents, such as ferumoxsil and barium sulfate, improve visualization and are generally considered safe in pregnancy.6 However, they have since fallen out of favor at many institutions with improved MR imaging techniques making them unnecessary to fully visualize the appendix. Similarly, intravenous gadolinium contrast aids detection of acute appendicitis and many of its pathological mimickers, but is generally avoided in pregnancy due to uncertain teratogenic risk. Gadolinium readily crosses the placenta into fetal circulation, although no adverse effects to mother or fetus have been described in the literature thus far.7 The American College of Radiology currently recommends that gadolinium contrast not be used routinely in pregnant patients and reserved only in situations where the potential benefit outweighs the risk.8
Our institution utilizes a 1.5-Tesla MR system with phased array body coil for the evaluation of abdominal pain in the pregnant patient. Informed consent is obtained and the patient is imaged in the supine position. We do not routinely administer oral or intravenous contrast. Our multiplanar MR imaging protocol consists of axial and coronal T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE), axial T2-weighted HASTE with fat saturation, axial T1-weighted gradient echo sequences (GRE) in and out-of-phase, axial T1-weighted GRE with fat saturation, and axial diffusion-weighted imaging (DWI).
Normal appendix
The normal appendix is a blind-ending tubular structure arising from the medial aspect of the cecal base, 1-3 cm below the ileocecal valve.9 It is classically described having an average length of 10 cm, wall thickness less than 2 mm, and total diameter less than 6 mm.9 However, recent studies have demonstrated up to 42% of normal appendices were greater than 6 mm in diameter when filled with intraluminal air.9,10 The distal appendix is often free-moving within the peritoneal cavity and presents in different orientations, with the pelvic, retrocecal, and post-ileal positions accounting for over 90% of cases.11 An appendix in the pelvic position lies in the peritoneal cavity pointing inferomedially towards the pelvis. A retrocecal appendix is located posterior to the cecum and is frequently a retroperitoneal structure. The post-ileal appendix is found extending posterior to the terminal ileum within the peritoneal cavity. The second and third trimester gravid uterus may also displace the appendix superiorly into the right upper quadrant, up to the level of the L2-L3 vertebral bodies.12,13
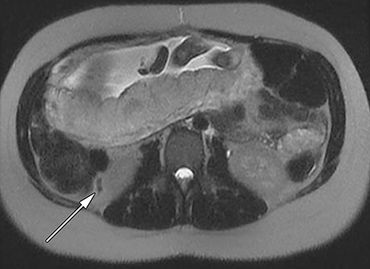
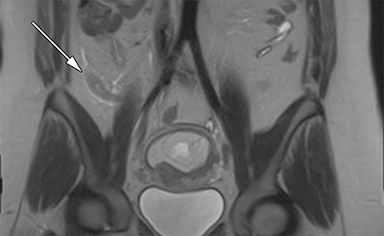
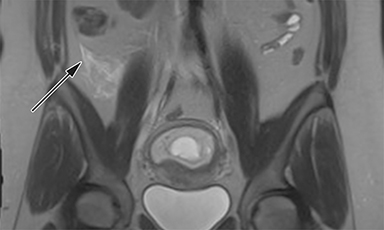
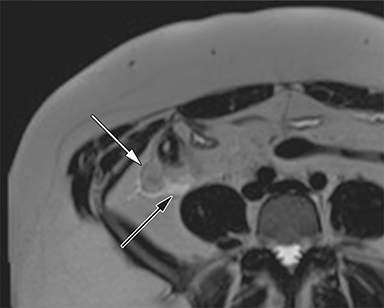
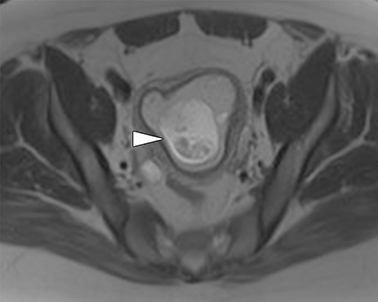
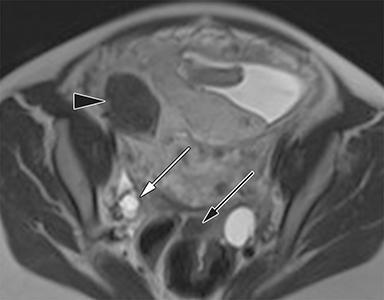
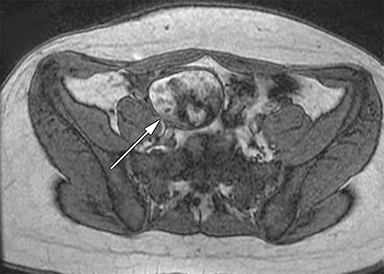
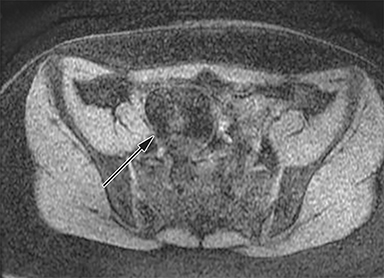
The normal appendix is filled with air and/or oral contrast without evidence of periappendiceal edema or inflammation. It has low signal intensity, approximating that of skeletal muscle on both T1 and T2-weighted MR imaging (Figure 1).14,15 Intraluminal air in the normal appendix may also be detected with the blooming effect, which produces a distinctly hypointense appendix on T2* time-of-flight and T1-weighted GRE in-phase imaging.13,15 The normal appendix is surrounded by periappendiceal fat of normal signal intensity with no evidence of edema or inflammation.16 Direct visualization of a normal appendix without periappendiceal inflammatory changes by MR imaging virtually excludes acute appendicitis in the pregnant patient.13
Acute appendicitis
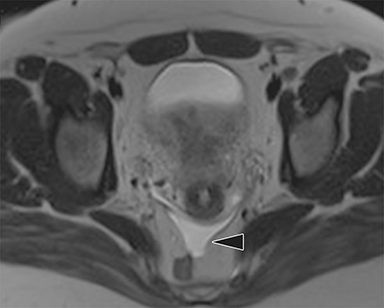
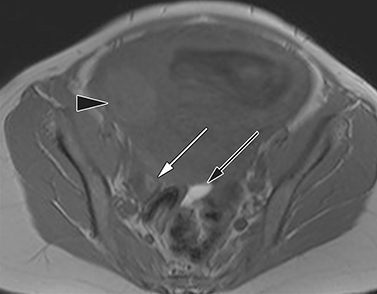
MR imaging findings suggestive of acute appendicitis include an enlarged, T2 hyperintense fluid-filled appendix with wall thickening and surrounding T2 hyperintensity representing periappendiceal edema or inflammation (Figure 2).15
Appendiceal diameter greater than 6 mm and wall thickness greater than 2 mm are commonly cited criteria suggesting acute inflammation.13 However, an appendiceal diameter greater than 6 mm in isolation of additional imaging findings was positive for acute appendicitis in only 31% of suspected cases.10 Appendiceal wall thickness is also difficult to accurately measure within the spatial resolution limitations of MR imaging and considerable discrepancy has been noted when assessing for wall thickening.10 Filling defects within a T2 hyperintense fluid-filled appendix may be seen and represents either appendicoliths or intraluminal air.15 Periappendiceal fat stranding is an important early sign of appendicitis, appearing as thin bands of T2 hyperintense fluid in the RLQ. Advanced cases may develop a periappendiceal phlegmon or abscess, appearing as a heterogeneous mass of moderate to high signal intensity on T2-weighted imaging, as well as ascites in the dependent portions of the abdomen and pelvis.15 If gadolinium contrast is used, the presence of a thickened enhancing appendiceal wall is further suggestive of acute appendicitis.14 The addition of DWI is becoming increasingly commonplace to improve detection and sensitivity, as 98.7% of acute appendicitis cases demonstrate high signal intensity or restricted diffusion and are characteristic of an acute inflammatory process.17,18
Visualization of the normal appendix is necessary to definitely exclude acute appendicitis, and is achieved in 78-90% of non-pregnant patients19,20 and 87% of pregnant women21,22 with MR imaging. In contrast, ultrasound is able to visualize the normal appendix in as few as 2% of pregnant women.21 With MR imaging, non-visualization of the appendix with no evidence of periappendiceal inflammation is considered an indeterminate finding, but has been shown to be negative for acute appendicitis in 94% of cases.23
Recent meta-analyses have demonstrated excellent sensitivity (90-91%) and specificity (98-99%) of MR imaging for the diagnosis of appendicitis during pregnancy.24,25 Performance is comparable to CT imaging (sensitivity,86%; specificity 97%)26 while avoiding exposure to ionizing radiation, making MR imaging the preferred adjunct to first-line ultrasound examination (sensitivity, 67-100%; specificity, 83-96%).27 Emergent use of MR imaging has been credited with reducing both the rate of unnecessary laparotomy and appendiceal rupture in pregnant patients.21
Mimickers of acute appendicitis
Fibroid degeneration
Fibroids, or leiomyomas, are the most common benign neoplasm of the uterus, with a prevalence of up to 25% in reproductive-age women and 3.9% in pregnant women.28 Fibroids are usually quiescent and asymptomatic during pregnancy, although up to 31% of fibroids undergo rapid growth during the first trimester from increases in maternal estrogen and progesterone.29,30 Red degeneration describes the hemorrhagic necrosis of a rapidly enlarging fibroid due to venous thrombosis of peripheral vessels or rupture of supplying arteries.29,31 Pelvic pain secondary to red degeneration is one the most common complication of fibroids during pregnancy.
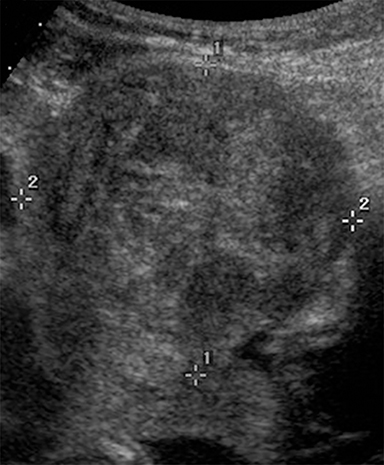
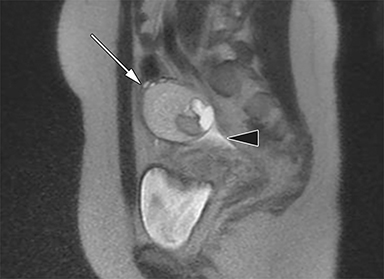
Although transvaginal ultrasound is the first-line modality for imaging uterine fibroids (Figure 3), MR imaging is superior in assessing the size, number and location of multiple fibroids in a large gravid uterus. Non-degenerated fibroids appear as well-circumscribed, homogenous masses that are distinctly T2 hypointense and T1 isointense compared to normal uterine myometrium.32, 33 Conversely, fibroids undergoing red degeneration demonstrate heterogeneous T1 and T2-weighted hyperintensity depending on the degree of hemorrhage and necrosis (Figure 4).32 A peripheral rim of T1 hyperintensity or T2 hypointensity may also be visualized, representing acute hemorrhage from peripheral thrombosed vessels surrounding the mass.34
Hemorrhagic ovarian cysts
Hemorrhagic ovarian cysts typically originate from benign functional ovarian, follicular or corpus luteal cysts, that have failed to regress, hemorrhaged internally and subsequently prone to rupture. Follicular cysts are a common finding in nearly all women of reproductive age, while corpus luteal cysts are particularly prevalent in early pregnancy before regressing after 7-8 weeks gestational age.35 Functional ovarian cysts are usually asymptomatic, but a hemorrhagic cyst may cause pelvic pain secondary to rapid distension of the ovarian capsule or cyst rupture.
On MR imaging, follicular cysts are thin-walled (<3 mm) cystic structures between 3-8 mm in diameter, while thicker-walled cysts greater than 10 mm diameter are more likely corpus luteal cysts.35, 36 These functional ovarian cysts are filled with serous fluid and demonstrate homogenous T1 hypointensity and T2 hyperintensity, with well-defined T2 hypointense walls. Conversely, hemorrhagic ovarian cysts are often imaged in the subacute phase and are classically hyperintense on both T1 and T2-weighted imaging due to blood contents (Figure 5).35, 36 However, their MR imaging appearance is variable and dependent on the degree and age of hemorrhage. One study found only 36% of hemorrhagic cysts demonstrated any degree of T1 hyperintensity and the remaining 64% appeared uniformly T1 hypointense.37 This is a useful distinguishing feature from endometriomas, where over 93% appear predominantly hyperintense on T1-weighted imaging.38
Dermoid cysts
Dermoid cysts are benign ovarian teratomas and are one of the most common types of ovarian masses, representing 20-40% of all ovarian neoplasms.39 They are cystic masses comprised of at least two different types of germ layers among ectoderm, mesoderm, and endoderm. Thus, dermoid cysts contain a combination of adipose, sebaceous, calcific, osseous, and hair components. The Rokitansky nodule, a common feature of dermoid cysts, is a solid protuberance projecting into the cavity and demonstrates particularly dense collections of germ layer derivatives.40
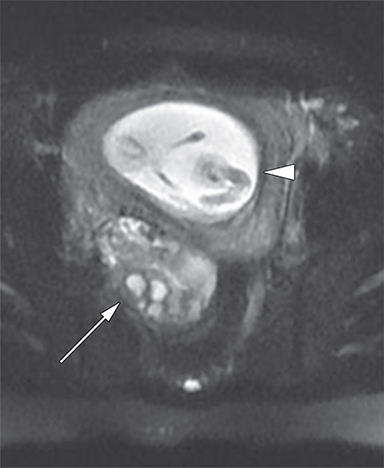
Dermoid cysts have a very characteristic appearance on MR imaging, as they most often contain adipose and calcific components, which are easily visualized. In addition, virtually all lipid-containing masses found in the ovaries are dermoid cysts.41 Adipose tissue and calcifications of teeth are found in 93% and 56% of dermoid cysts respectively.42 They typically present as large cystic structures, average diameter of 7 cm, with heterogeneous signal intensity. The adipose or sebaceous component is visualized as T1 hyperintense with signal dropout on fat suppression sequences, differentiating it from hemorrhagic masses, which are also typically T1 hyperintense (Figure 6). Uncommonly, dermoid cysts may be fluid-filled and contain only mural microscopic fat, in which case it is helpful to look for fat signal dropout on T1-weighted out-of-phase images.43 Teeth, bone, hair, serous fluid, and other calcifications all appear hypointense with T1-weighted imaging. The appearance of adipose tissue with T2-weighted imaging is more variable, but is typically hyperintense relative to skeletal muscle.41
Endometriomas
Endometriosis describes the presence of functional endometrial tissue implanted outside the uterus. It is believed to affect up to 10% of reproductive-age women, but only 2.5% of pregnancies as it is associated with higher rates of female infertility.44 However, with increasing prevalence and efficacy of assisted reproductive therapies, the incidence of endometriosis in pregnancy appears to be rising.45 Endometriosis affects the ovaries as endometriomas, but may also produce peritoneal implants and adhesions involving the uterine ligaments, peritoneum, bowel, and bladder.46
Endometriomas are cyst-like structures with an interior lining of ectopic endometrial tissue that has implanted within the ovary. Endometriomas are also known as “chocolate cysts” as the proliferating endometrial tissue continues to produce a chocolate-colored mixture of blood products within the cyst cavity. Endometriomas represent 11% of ovarian masses detected in pregnancy.47 In pregnant women, endometriosis and endometriomas may be asymptomatic, or may present with pelvic pain as a result of peritoneal inflammation and formation of adhesions.48
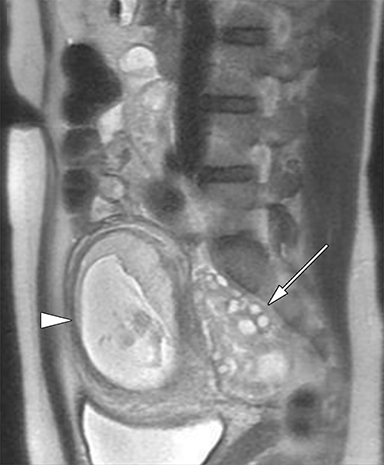
The key MR findings of an endometrioma is an ovarian cyst with T1 hyperintensity with characteristic T2 shading (Figure 7).38 Endometriomas classically demonstrate uniform T1 hyperintensity from high concentrations of paramagnetic hemoglobin within the cyst cavity.49 T2 shading describes low signal intensity on T2-weighted imaging as a result of blood products such as methemoglobin, protein, and iron that accumulates as endometriomas repeatedly hemorrhage.50 Presence of multiple internal T1 hyperintense foci (multiplicity) is highly suggestive of an endometrioma, reflecting multiple cycles of internal hemorrhage and rupture of endometriotic cysts.38,49 Endometriomas may respond to increases in progesterone and undergo decidualization. This produces T2 hyperintense mural nodules, which are often prominent within the typically T2 hypointense cyst cavity.50
Ovarian torsion
Ovarian torsion, a true gynecological emergency, describes the twisting of an ovary around its ligamentous support, compromising its vascular supply and leading to irreversible ischemic necrosis. It is relatively uncommon at an incidence of approximately 1/1000 pregnancies, but may affect upwards of 16% of pregnancies achieved through ovarian hyperstimulation.51 Ovarian torsion occurs most commonly in the 1st and 2nd trimesters, attributable to increased ligamentous laxity, rapid uterine growth, and a greater number of functional cysts present in early pregnancy.51
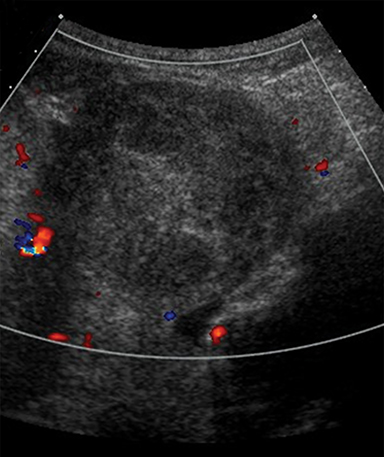
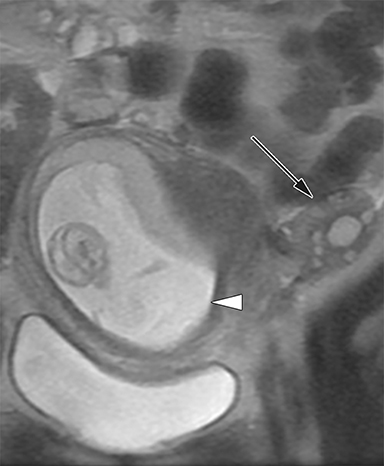
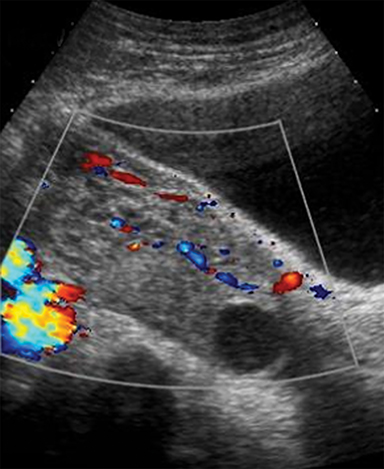
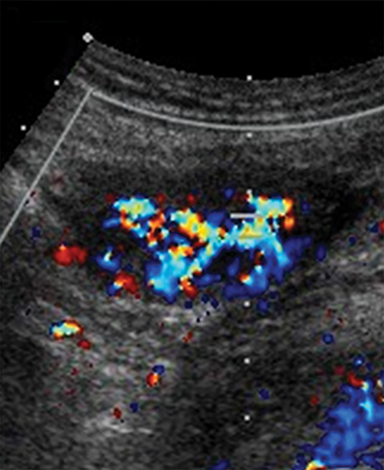
MR imaging features of a torsed ovary includes an enlarged ovary greater than 4 cm in diameter, stromal edema or hemorrhage, peripherally located cysts, tubal thickening, a twisted vascular pedicle, and ipsilateral uterine deviation.52 Stromal edema presents as diffuse T2-weighted hyperintensity, best appreciated with fat-suppression sequences and when compared to a normal contralateral ovary (Figure 8).15 Acute to subacute ovarian hemorrhage manifests as a peripheral rim of hyperintensity on T1-weighted imaging and hypointensity on T2-weighted imaging.52 Peripherally located follicles are best appreciated on T2-weighted imaging and deviation of the uterus towards the torsed ovary was noted in 36-42% of cases.53, 54 Ascites and hemoperitoneum are less common findings that may be present depending on the degree and chronicity of torsion.53 Ultrasound is the typical initial imaging modality for suspected ovarian torsion in pregnant patients. Sonographic findings are similar and include a unilaterally enlarged ovary with an associated mass, peripheral located cysts, a twisted vascular pedicle, and poor vascular perfusion on Doppler ultrasound (Figure 9).55
Conclusion
Assessment of RLQ pain and suspected appendicitis in the pregnant patient is made difficult by changes in maternal physiology, restrictions to diagnostic imaging, and a wide differential of disease mimickers. MR imaging is a safe and effective investigation in pregnancy to exclude acute appendicitis and simultaneously assess alternative genitourinary causes. Familiarity with the key MR imaging findings of acute appendicitis and its disease mimickers allows for accurate and prompt assessment of RLQ pain in the pregnant patient in an emergency setting.
References
- Mourad J, Elliott JP, Erickson L, et al. Appendicitis in pregnancy: new information that contradicts long-held clinical beliefs. Am J Obstet Gynecol. 2000;182(5):1027-1029.
- Ueberrueck T, Koch A, Meyer L, et al. Ninety-four appendectomies for suspected acute appendicitis during pregnancy. World J Surg. 2004;28(5):508-511.
- Yilmaz HG, Akgun Y, Bac B, et al. Acute appendicitis in pregnancy — risk factors associated with principal outcomes: a case control study. Int J Surg. 2007;5(3):192-197.
- Cappell MS, Friedel D. Abdominal pain during pregnancy. Gastroenterol Clin North Am. 2003;32(1):1-58.
- Smith MP, Katz DS, Rosen MP, et al. ACR Appropriateness Criteria® right lower quadrant pain—suspected appendicitis. Available at https://acsearch.acr.org/docs/69357/Narrative/. American College of Radiology. Accessed Jan 1, 2016.
- Han BH, Lee KS, Han JY, et al. Pregnancy outcome after 1st-trimester inadvertent exposure to barium sulphate as a contrast media for upper gastrointestinal tract radiography. J Obstet Gynaecol. 2011;31(7):586-588.
- Webb JAW, Thomsen HS, Morcos SK. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005;15(6):1234-1240.
- Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37(3):501-530.
- Dewhurst C, Beddy P, Pedrosa I. MRI evaluation of acute appendicitis in pregnancy. J Magn Reson Imaging. 2013;37(3):566-575.
- Tamburrini S, Brunetti A, Brown M, et al. CT appearance of the normal appendix in adults. Eur Radiol. 2005;15(10):2096-2103.
- Ahmed I, Asgeirsson KS, Beckingham IJ, et al. The position of the vermiform appendix at laparoscopy. Surg Radiol Anat. 2007;29(2):165-168.
- Baer J, Reis R, Araens R. Appendicitis in pregnancy: with changes in position and axis of the normal appendix in pregnancy. JAMA. 1932;98(16):1359-1364.
- Pedrosa I, Levine D, Eyvazzadeh AD, et al. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3)891-899.
- Singh A, Danrad R, Hahn PF, et al. MR imaging of the acute abdomen and pelvis: acute appendicitis and beyond. Radiographics. 2007;27(5):1419-1431.
- Pedrosa I, Zeikus EA, Levine D, et al. MR imaging of acute right lower quadrant pain in pregnant and nonpregnant patients. Radiographics. 2007;27(3):721-743.
- Spalluto LB, Woodfield CA, DeBenedectis CM, et al. MR imaging evaluation of abdominal pain during pregnancy: appendicitis and other nonobstetric causes. Radiographics. 2012;32(2):317-334.
- Inci E, Kilickesmez O, Hocaoglu E, et al. Utility of diffusion-weighted imaging in the diagnosis of acute appendicitis. Eur Radiol. 2011;21(4):768-775.
- Leeuwenburgh MM, Wiarda BM, Bipat S, et al. Acute appendicitis on abdominal MR images: training readers to improve diagnostic accuracy. Radiology. 2012;264(2):455-463.
- Nikolaidis P, Hammond N, Marko J, et al. Incidence of visualization of the normal appendix on different MRI sequences. Emerg Radiol. 2006;12(5):223-226.
- Nitta N, Takahashi M, Furukawa A, et al. MR imaging of the normal appendix and acute appendicitis. J Magn Reson Imaging. 2005;21(2):156-165.
- Pedrosa I, Lafornara M, Pandharipande PV, et al. Pregnant patients suspected of having acute appendicitis: effect of MR imaging on negative laparotomy rate and appendiceal perforation rate. Radiology. 2009;250(3):749-757.
- Oto A, Ernst RD, Shah R, et al. Right lower quadrant pain and suspected appendicitis in pregnant women: evaluation with MR imaging—initial experience. Radiology. 2005;234(2):445-451.
- Israel GM, Malguria N, McCarthy S, et al. MRI vs. ultrasound for suspected appendicitis during pregnancy. J Magn Reson Imaging. 2008;28(2):428-433.
- Long SS, Long C, Lai H, et al. Imaging strategies for right lower quadrant pain in pregnancy. AJR Am J Roentgenol. 2011;196(1):4-12.
- Blumenfeld YJ, Wong AE, Jafari A, et al. MR imaging in cases of antenatal suspected appendicitis – a meta-analysis. J Matern Fetal Neonatal Med. 2011;24(3):485-488.
- Basaran A, Basaran M. Diagnosis of acute appendicitis during pregnancy. Obstet Gynecol Surv. 2009;64(7):481-488.
- Williams R, Shaw J. Ultrasound scanning in the diagnosis of acute appendicitis in pregnancy. Emerg Med J. 2007;24(5):359-360.
- Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107(2 Pt 1):376-382.
- Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87(4):725-736.
- Rosati P, Exacoustòs C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy: a sonographic study. J Ultrasound Med. 1992;11(10):511-515.
- Zaima A, Ash A. Fibroid in pregnancy: characteristics, complications, and management. Postgrad Med J. 2011;87(1034):819-828.
- Deshmukh SP, Gonsalves CF, Guglielmo FF, et al. Role of MR imaging of uterine leiomyomas before and after embolization. Radiographics. 2012;32(6):E251-E281.
- Ueda H, Togashi K, Konishi I, et al. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiographics. 1999;19(Spec No):S131-S145.
- Kawakami S, Togashi K, Konishi I, et al. Red degeneration of uterine leiomyoma: MR appearance. J Comput Assist Tomogr. 1994;18(6):925-928.
- Togashi K. MR imaging of the ovaries: normal appearance and benign disease. Radiol Clin North Am. 2003;41(4):799-811.
- Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. Radiographics. 2000;20(5):1445-1470.
- Kanso HN, Hachem K, Aoun NJ, et al. Variable MR findings in ovarian functional hemorrhagic cysts. J Magn Reson Imaging. 2006;24(2):356-361.
- Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: diagnosis with MR imaging. Radiology. 1991;180(2):73-78.
- Ayhan A, Bukulmez O, Genc C, et al. Mature cystic teratomas of the ovary: case series from one institution over 34 years. Eur J Obstet Gynecol Reprod Biol. 2000;88(2):153-157.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475-490.
- Saba L, Guerriero S, Sulcis R, et al. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur J Radiol. 2009;72(3):454-463.
- Buy JN, Ghossain MA, Moss AA, et al. Cystic teratoma of the ovary: CT detection. Radiology. 1989;171(3):697-701.
- Yamashita Y, Hatanaka Y, Torashima M, et al. Mature cystic teratomas of the ovary without fat in the cystic cavity: MR features in 12 cases. AJR Am J Roentgenol. 1994;163(3):613-616.
- Aris A. A 12-year cohort study on adverse pregnancy outcomes in eastern townships of Canada: impact of endometriosis. Gynecol Endocrinol. 2014;30(1):34-37.
- Ueda Y, Enomoto T, Miyatake T, et al. A retrospective analysis of ovarian endometriosis during pregnancy. Fertil Steril. 2010;94(1):78-84.
- Gougoutas CA, Siegelman ES, Hunt J, et al. Pelvic endometriosis: various manifestations and MR imaging findings. AJR Am J Roentgenol. 2000;175(2):353-358.
- Poder L, Coakley FV, Rabban JT, et al. Decidualized endometrioma during pregnancy: recognizing an imaging mimic of ovarian malignancy. J Comput Assist Tomogr. 2008;32(4):555-558.
- Khan KN, Kitajima M, Fujishita A, et al. Pelvic pain in women with ovarian endometrioma is mostly associated with coexisting peritoneal lesions. Hum Reprod. 2013;28(1):109-118.
- McDermott S, Oei TN, Iyer VR, et al. MR imaging of malignancies arising in endometriomas and extraovarian endometriosis. Radiographics. 2012;32(3):845-863.
- Siegelman ES, Oliver ER. MR imaging of endometriosis: ten imaging pearls. Radiographics. 2012;32(6):1675-1691.
- Smorgick N, Pansky M, Feingold M, et al. The clinical characteristics and sonographic findings of maternal ovarian torsion in pregnancy. Fertil Steril. 2009;92(6):1983-1987.
- Duigenan S, Oliva E, Lee SI. Ovarian torsion: diagnostic features on CT and MRI with pathologic correlation. AJR Am J Roentgenol. 2012;198(2):W122-W131.
- Rha SE, Byun JY, Jung SE, et al. CT and MR imaging features of adnexal torsion. Radiographics. 2002;22(2):283-294.
- Harmon JC, Binkovitz LA, Stephens J. Uterine position in adnexal torsion: specificity and sensitivity of ipsilateral deviation of the uterus. Pediatr Radiol. 2009;39(4):354-358.
- Chang HC, Bhatt S, Dogra VS. Pearls and pitfalls in diagnosis of ovarian torsion. Radiographics. 2008;28(5):1355-1368.
Citation
AA H, KA M, R P, SD C, AC H. MRI of right lower quadrant pain in pregnancy: Appendicitis and mimickers. Appl Radiol. 2017;(12):6-13.
December 7, 2017