Obscure GI bleeding: The role of multiphase CT enterography


Dr. Huprich is an Associate Professor of Radiology, Dr. Fletcher is a Professor of Radiology, Dr. Fidler is an Associate Professor of Radiology, and Mr. Llano is a Research Fellow, Department of Radiology, Mayo Clinic, Rochester, MN.
Dr. Huprich and Dr. Fidler reported receiving research support from Bracco Diagnostics and Dr. Fletcher reported receiving research support from Siemens Healthcare.
Obscure GI bleeding (OGIB), defined as persistent or recurrent GI bleeding despite negative initial upper endoscopy and colonoscopy, is one of the most challenging problems facing the practicing clinician.1 In most cases the presumed cause for the blood loss is a small bowel abnormality. Until recently, results from conventional imaging techniques designed to detect small bowel bleeding sources were disappointing. Introduced in 2000, wireless capsule endoscopy (CE) has rapidly become the preferred technique for the evaluation of OGIB. CE has a diagnostic yield of 32% to 76%, and a negative predictive value of 83% to 100%.2 However, CE is not a perfect test. In one report, complete examination of the small bowel was achieved in only 74% of patients and trivial findings were seen in 23%.3 Capsule retention requiring endoscopic or surgical retrieval occurs in 1.9% .4 In addition, the procedure is contraindicated in patients with pacemakers, dysphagia, and suspected small bowel strictures. Because of the transit time through the GI tract and the huge number of images to be reviewed, long turnaround times are common. This fact makes it unsuitable for the evaluation of acute severe OGIB, in which early diagnosis is important.
Multiple reports of the use of computed tomography (CT) in evaluating GI bleeding have been published.5 Most emphasize the detection of active bleeding. However, as we have learned from angiographic and nuclear medicine studies, which are designed to detect brisk active bleeding, bleeding tends to be intermittent. Therefore, if multiphase CT enterography (MpCTE) is performed between bleeding episodes, it will be falsely negative. Furthermore, the majority of CT studies do not emphasize detection of the etiology of the bleeding. Prompt determination of the bleeding etiology may be important in directing treatment and predicting a patient’s prognosis. Patients with small bowel angiodysplasias will require different treatment and usually have a better prognosis than those with potentially malignant small bowel neoplasms.
Encouraged by the success of single-phase CT enterography in the evaluation of inflammatory bowel disease and the exquisite images it produces,6 we modified the single-phase enterography technique to enhance our ability to detect lesions responsible for small bowel bleeding and introduced it in our practice in 2006. Our gastroenterology colleagues have found this technique very useful and it has become the initial diagnostic tool in the evaluation of obscure GI bleeding. The purposes of this paper are to describe the technical aspects of MpCTE, to present the imaging findings, and to discuss performance estimates in patients with obscure GI bleeding.
MpCTE technique and interpretation methods
Patients referred for MpCTE should have evidence of persistent or recurrent GI blood loss (iron deficiency anemia and/or positive fecal occult blood test with or without hematochezia or melena) and should have undergone recent negative upper endoscopy and colonoscopy.
Because of the high spatial and temporal resolution required, these exams should be performed on a 64- or 128-slice CT system. After a 4-hour fast, patients are given a total of 1350 cc of 0.1% w/v barium sulfate suspension (VoLumen®, E-Z-EM, Lake Success, NY) orally, divided into three 450-cc doses given every 15 minutes, beginning 60 minutes prior to scanning. An additional 500 cc of water is given orally 15 minutes before scanning. One hundred fifty cc of Omnipaque 300® (GE Healthcare, Ltd, distributed by Amersham Health, Princeton, NJ) is power injected intravenously through an antecubital catheter at a rate of 4 cc/second. Scanning is performed from the diaphragm to the symphysis pubis during each of 3 phases: (1) a bolus-triggered “arterial phase;” (2) an “enteric phase” beginning 50 sec after the beginning of injection; and (3) a “delayed phase,” 90 sec after the beginning of contrast injection, using a 64-slice or 128-slice CT system. Bolus triggering is performed by the CT technologist by placing an ROI cursor over the descending aorta 2 cm above the diaphragm. The ROI trigger threshold is set at 150 HU at 120 kVp, with scanning initiated 6 seconds after the threshold CT number is achieved. Patients are scanned at 120 kVp with quality reference effective mAs of 240 mAs. Detector collimation is set to the minimum slice width. For each phase of acquisition, axial images are reconstructed using a 2-mm slice width and 1-mm reconstruction interval. Coronal-multiplanar images are reconstructed from the retroperitoneal border to the anterior abdominal wall at 2-mm slice width and 1-mm reconstruction interval.
Interpretation of these studies can be very challenging. Two-millimeter slices in the axial and coronal planes covering the entire abdomen and pelvis, repeated for 3 phases, can generate 1500-2500 images per case. Lesions may consist of a tiny nidus of enhancement visible on only 1 of the 3 phases, which adds an additional challenge. Also, some masses may be isodense with the bowel wall and completely undetectable if the bowel is under-distended. To maximize the chances for successful interpretation, it is necessary to have a strategy for viewing these studies. We read all our studies on a dual-monitor workstation. Our method of review consists of placing the enteric phase on the left monitor and the arterial and delayed images on the right -- one above the other. All images are synchronized so that identical anatomic locations are displayed while we scroll through the images from each phase. Viewing each phase simultaneously allows the appreciation of the evolving nature of enhancing lesions. Once a suspicious area is found, the corresponding co-registered coronal images are displayed to determine the approximate location and lesion shape.
While this technique is designed to detect small bowel abnormalities responsible for bleeding, the entire GI tract should be examined. We know from previous reports that endoscopy fails to detect endoscopically accessible lesions in the upper GI tract in up to 26% of patients.7 It is therefore important when interpreting MpCTE exams not only to evaluate the small bowel, but also to carefully examine the upper GI tract and colon. A poorly prepared colon may prevent detection of a lesion at colonoscopy which may be visible on MpCTE. Furthermore, submucosal lesions may not produce mucosal disruption and, therefore, they may go undetected by endoscopy.
MpCTE findings in OGIB
The list of small-bowel abnormalities responsible for OGIB is long. The source of small-bowel bleeding varies somewhat, depending upon the patient’s age. In patients younger than 40 years, small-bowel tumors, Crohn’s disease, Meckel’s diverticulum, and Dieulafoy lesions are more prevalent than in older patients. In patients older than 50 years, vascular lesions make up 40% of etiologies, followed by NSAID-induced small bowel disease, and a variety of less common disorders.8
Lesions responsible for OGIB may be visible on MpCTE as focal areas of enhancement that may vary in intensity on each phase, depending upon the nature of the abnormality. Others may appear as mass lesions that may or may not enhance. Isodense polypoid masses may be undetectable when concealed by incompletely distended bowel. Isodense lesions, small lesions, and lesions visible on only 1 phase or the 3 phases are the most frequently overlooked.
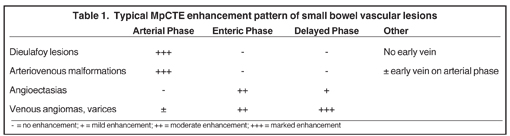
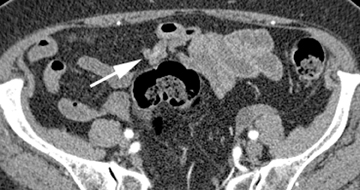
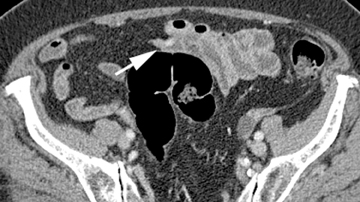
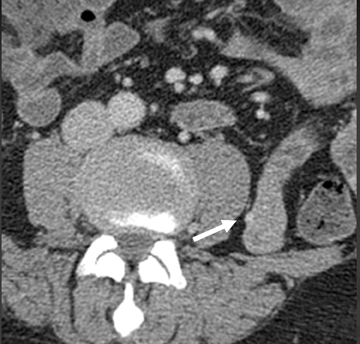
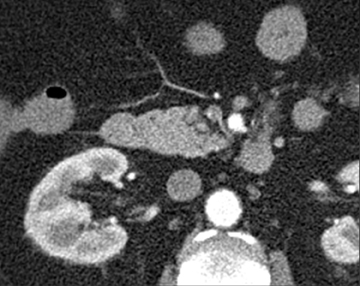
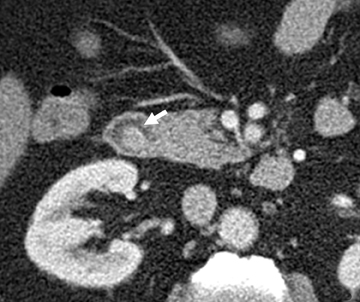
The shape, enhancement characteristics, and evolving appearance of the lesion during the 3 phases may allow a specific diagnosis, expediting treatment decisions. For example, a tiny nidus of bright enhancement, evident only on the arterial phase, with an associated early-draining mesenteric vein, likely represents an arteriovenous malformation and may be treated with embolization (Figure 1). On the other hand, a small-bowel carcinoid, appearing as a plaque-like enhancing intramural mass, brightest on the enteric phase and fading slightly on the delayed phase, should trigger a surgical consult (Figure 2).
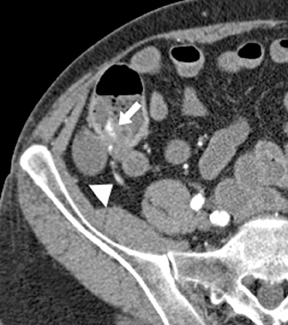
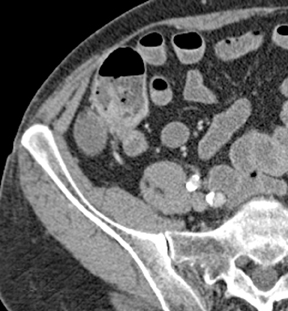
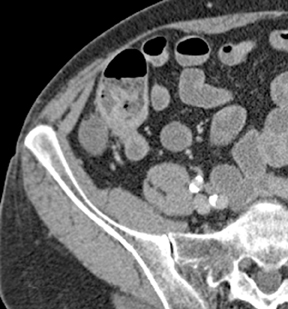
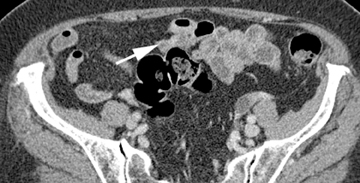
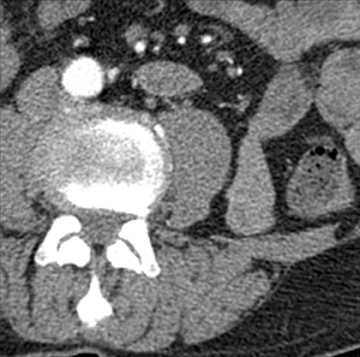
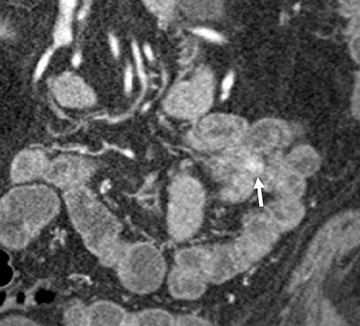
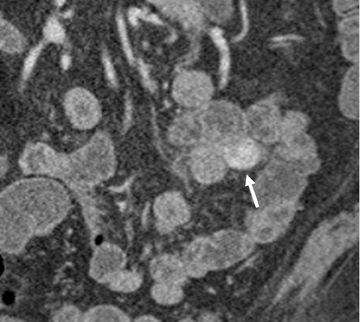
Small-bowel vascular lesions consist of angioectasias, Dieulafoy lesions, arteriovenous malformations (AVMs), venous angiomas and varices.9 The MpCTE enhancement pattern of small-bowel vascular lesions is frequently characteristic (Table 1). Angioectasias are the most common type; they may appear as tiny nodular or plaque-like areas of enhancement brightest on the enteric phase, fading slightly on the delayed phase (Figure 3), or as segments of bulbous swelling within intramural vessels (Figure 4). This latter appearance is often seen in the jejunum in older patients with no history of OGIB. Boley described dilated intramural veins in association with colonic angioectasia.10 Whether this finding is associated with small-bowel angioectasias or it represents a normal finding is uncertain. Due to the high flow, Dieulafoy lesions and AVMs may be visible only on the arterial phase and disappear on subsequent phases. Unlike Dieulafoy lesions, AVMs are sometimes accompanied by an early draining vein.
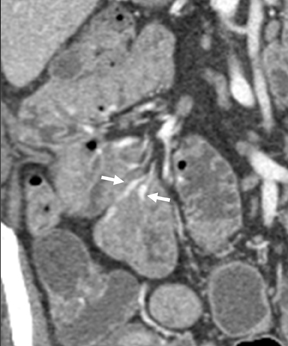
Venous angiomas of the small bowel are rare. Their MpCTE appearance resembles hepatic cavernous hemangiomas—demonstrating progressive enhancement during subsequent phases. Ectopic varices in the small intestine (Figure 5) represent portal-systemic shunts; they usually occur in association with adhesions between the small intestine and adjacent structures, such as the abdominal wall, which develop as a result of prior operations or abdominal injury.
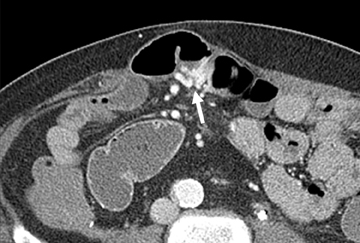
In our experience, small-bowel carcinoids are one of the most common neoplasms encountered in OGIB. When small, they may appear as a nodule or plaque-like area of enhancement in the bowel wall, brightest on the enteric phase and fading slightly on the delayed images. The serosal surface may be slightly retracted because of fibrotic reaction associated with these tumors (Figure 6). Associated mesenteric adenopathy is common, even with small tumors, and should trigger a closer look for an enhancing bowel wall mass.
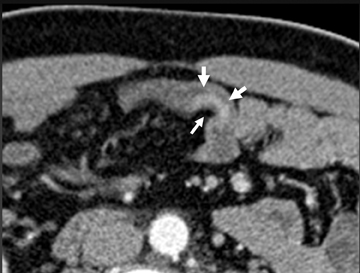
A variety of other small-bowel masses may be encountered. Often these lesions may be small and isodense with bowel wall, making them very difficult to detect. In addition, without adequate bowel distention, polypoid lesions will be shrouded by the collapsed bowel wall and go undetected. Often, the bowel loop will be distended enough during 1 or 2 of the phases to permit detection of the mass (Figure 7). Sometimes an isodense lesion may be more obvious on coronal or sagittal images.
Active bleeding is manifested on MpCTE as the progressive accumulation of intraluminal contrast displayed over the 3 phases. The extravasated contrast may pool on the dependent surface of the bowel or it may be dispersed by peristalsis into a more irregular shape. Active bleeding should be suspected whenever one sees a contrast collection that expands in size over subsequent phases (Figure 8). Compared to prior reports on the use of CT in GI bleeding, in our practice, which consists principally of hemodynamically stable outpatients, active bleeding is relatively uncommon. The ability of MpCTE to detect non-bleeding small bowel lesions responsible for OGIB is a testimony to the robustness of this technique.
Performance of MpCTE in obscure GI bleeding
We introduced MpCTE into our practice in 2005. We reported our initial experience in a retrospective study of 22 patients with OGIB who underwent multiphase CT enterography using the technique described above. CT findings were compared with capsule and traditional endoscopy, surgery, and angiography. MpCTE was positive for a bleeding source in 10 of 22 patients (45%). Eight of 10 positive MpCTE exams agreed with capsule endoscopy findings or subsequent final diagnosis. MpCTE correctly identified 3 lesions undetected at capsule endoscopy. The results of the study suggested an important role in evaluating obscure GI bleeding.11 In a follow-up blinded, prospective study conducted at our institution comparing MpCTE with capsule endoscopy in a group of patients with obscure GI bleeding, MpCTE detected a small-bowel bleeding source in 14 of 16 patients (88%) compared to 6 of 16 (38%) for CE. MpCTE detected all 9 small-bowel masses compared to 3 of 9 for CE.12 As a result of these studies, MpCTE has become the initial preferred exam in our practice for the workup of patients with OGIB. We currently perform 20 to 30 MpCTE exams each month. Patients with negative MpCTE exams usually undergo capsule endoscopy.
Patient considerations
The major drawback of MpCTE is the significant patient radiation dose (equivalent to 3 single-phase CT-enterography exams—approximately 35 to 40 mSv). Obviously, the dose can be reduced by eliminating 1 or 2 of the phases, but based upon our previous study, doing so significantly lowers sensitivity.13 The recent introduction of dose reduction techniques promises to offer significantly reduced patient dose without loss of diagnostic accuracy.14 Furthermore, we should apply this technique judiciously by insisting that patients undergo upper endoscopy and colonoscopy to exclude a bleeding source prior to performing MpCTE. In younger patients whose cumulative lifetime radiation risk is likely to be greater than older patients, one must be even more cautious so as not to expose them to unnecessary risks of radiation.
Conclusion
MpCTE is a robust technique for the evaluation of OGIB. It can be performed easily in routine clinical settings. Combined with capsule endoscopy, it offers the best chance for detecting the cause of obscure GI bleeding.
References
- Raju GS, Gerson L, Das A, Lewis B, American Gastroenterological A. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717.
- Concha R, Amaro R, Barkin JS. Obscure gastrointestinal bleeding: Diagnostic and therapeutic approach. J Clin Gastroenterol. 2007;41:242-251.
- Leighton JA, Goldstein J, Hirota W, et al. Obscure gastrointestinal bleeding. Gastrointest Endosc. 2003;58:650-655.
- Rondonotti E, Villa F, Mulder CJJ, et al. Small bowel capsule endoscopy in 2007: Indications, risks and limitations. World J Gastroenterol. 2007;13:6140-6149.
- Wu L, Xu J, Yin Y, et al. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: A meta-analysis. World J Gastroenterol. 2010;16:3957-3963.
- Paulsen SR, Huprich JE, Fletcher JG, et al. CT enterography as a diagnostic tool in evaluating small bowel disorders: Review of clinical experience with over 700 cases. Radiographics. 2006;26:641-657; discussion 657-662.
- Zaman A, Katon RM. Push enteroscopy for obscure gastrointestinal bleeding yields a high incidence of proximal lesions within reach of a standard endoscope. Gastrointest Endosc. 1998;47:372-376.
- Lin S, Rockey DC. Obscure gastrointestinal bleeding. Gastroenterol Clin North Am. 2005; 34:679-698.
- Yano T, Yamamoto H. Vascular, polypoid, and other lesions of the small bowel. Best Pract Res Clin Gastroenterol. 2009;23:61-74.
- Boley SJ, Sammartano R, Adams A, et al. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology. 1977;72:650-660.
- Huprich J, Fletcher J, Alexander JA, et al. Obscure gastrointestinal bleeding: Evaluation with 64-section multiphase CT enterography -- initial experience. Radiology. 2008;246:562-571.
- Huprich JE, Fletcher JG, Fidler JL, et al. Prospective blinded comparison of wireless capsule endoscopy and multi-phase CT enterography in obscure GI bleeding. Radiology. 2011;260:744-751.
- Thacker P, Huprich J, Barlow J, et al. The performance of triple-phase CT enterography compared to single-phase and double-phase enterography in the evaluation of obscure GI bleeding. Presented at: Radiological Society of North America, 95th Scientific Assembly and Annual Meeting. Chicago, IL, 2009.
- McCollough CH Bruesewitz M, Kofler JM. CT dose reduction and dose management tools: Overview of available options. Radiographics. 2006;26:503-512.
