Ovarian Masses and O-RADS: A Systematic Approach to Evaluating and Characterizing Adnexal Masses with Ultrasound
By Mitchell A, Kwong A, Sekhon S, McGahan JP
Editor’s note: This is the first part of a two-part series. Part II will be presented in the July-August 2021 issue of Applied Radiology.
Clinical presentation and pertinent laboratory analysis often determine the appropriate imaging differential diagnostic considerations in evaluating anyone with ovaries presenting with pelvic symptoms. In patients with a positive pregnancy test, sonography is the modality of choice to evaluate the pregnancy and any complications. Sonography is also the modality of choice for evaluating patients who present with pelvic pain or a mass but are not pregnant. Pelvic sonography is the preferred imaging modality when an obstetrical or gynecologic etiology is suspected under American College of Radiology (ACR) Appropriateness Criteria.1
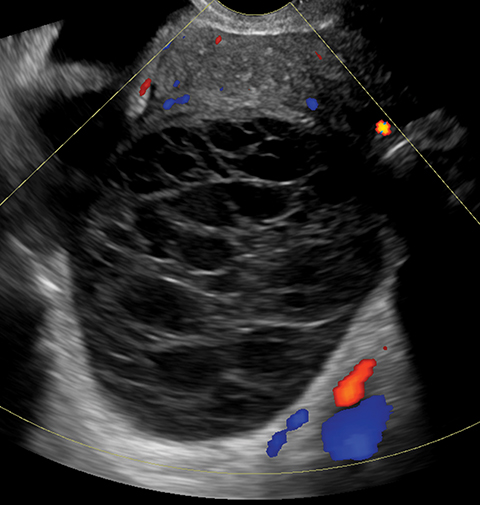
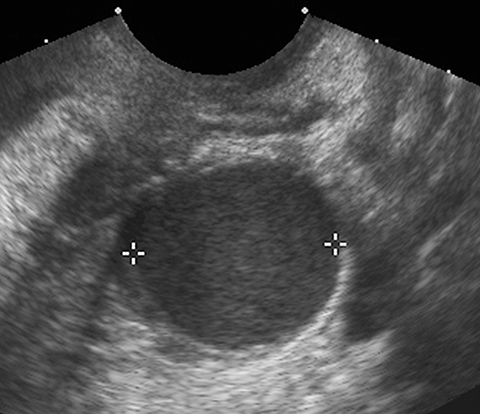
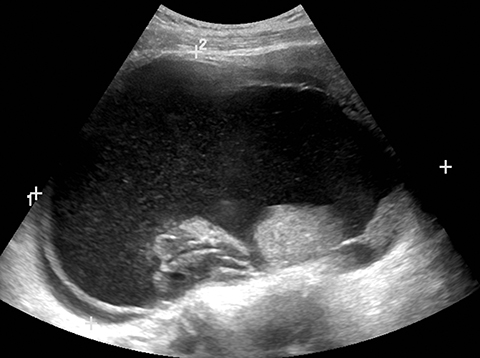
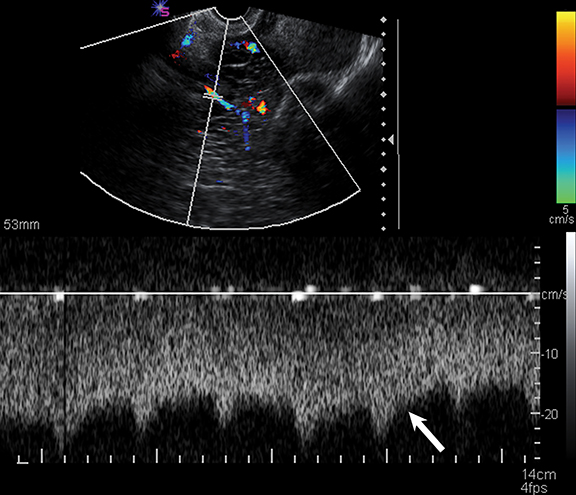
Sonography is useful in evaluating for the presence of an adnexal mass as well as for mass characterization.2 For example, the sonographic appearance of a retracting clot and/or a reticular pattern without Doppler flow/vascularity is diagnostic of a hemorrhagic cyst (Figure 1).3 Most adnexal cysts are benign and are easily characterized by ultrasound. However, evaluation of indeterminate adnexal masses by an ultrasound expert is highly valuable.3 Published data supports the use of pattern recognition by an experienced ultrasound examiner as a highly accurate method of discriminating between benign and malignant adnexal masses without the need for MRI.3 In particularly challenging cases, contrast-enhanced MRI may be helpful in differentiating between benign and malignant ovarian masses.
This two-part review describes a stepwise approach to evaluating and characterizing adnexal masses in non-pregnant patients. Here in part 1, we will review the utilization of ultrasound in this endeavor. In part 2, we will review the use of MRI.
Ultrasound Imaging
In evaluating any pelvic mass, the first step is to determine if the mass is arising from the ovaries, the uterus, or another location. Once the anatomical origin is determined, imaging may be extremely helpful in establishing an accurate diagnosis. If the mass is of ovarian origin, for example, identifying its nature—cystic, solid, or complex—and ascertaining the presence of any fat or calcium in the mass will helps narrow the differential diagnoses.
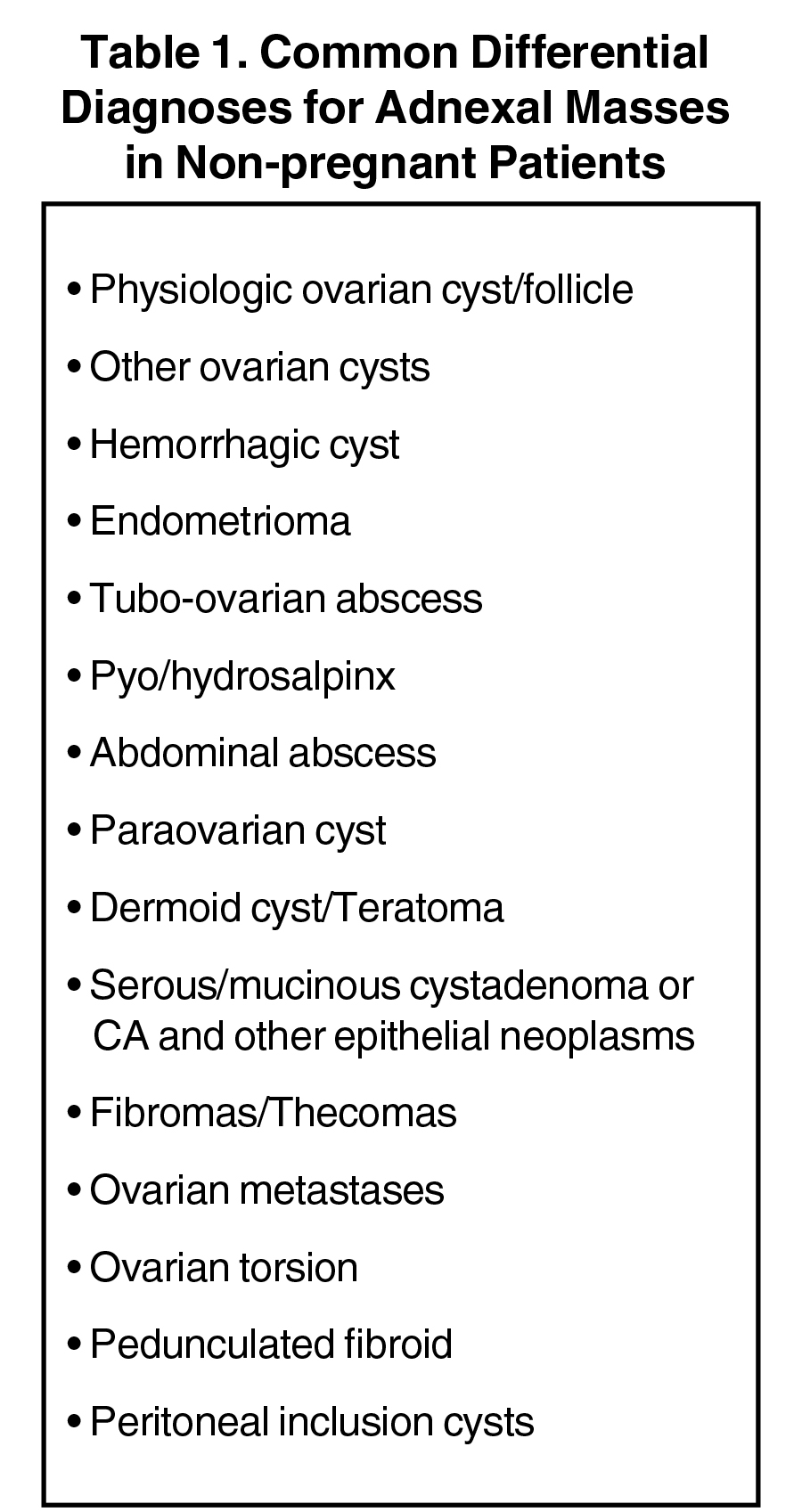
Ultrasound is also invaluable for determining the cystic or solid nature of adnexal masses. Although a comprehensive discussion of all adnexal masses is beyond the scope of this pictorial review, Table 1 provides a list of some common cystic and solid adnexal masses that may be detected, and in most cases diagnosed, with sonography. In 2019, the Society of Radiologists in Ultrasound (SRU) updated its 2010 consensus statement on the management of asymptomatic ovarian and adnexal cysts.2
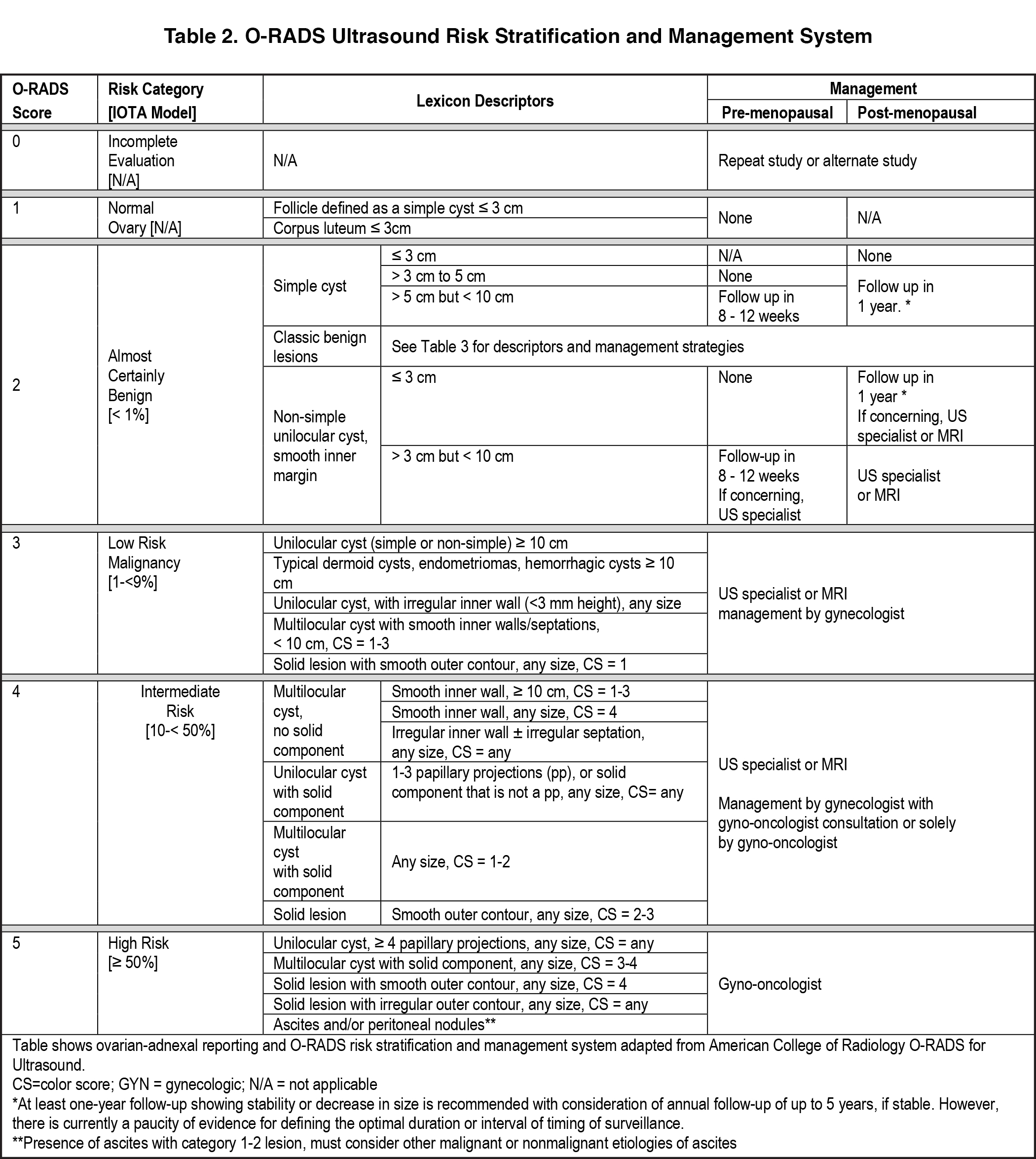
More recently, the ACR convened a consensus panel on risk stratification of ovarian-adnexal masses and management using an O-RADSTM classification system.3 This system is more comprehensive than the SRU system in that it not only makes recommendations for classifying simple cysts, but also includes recommendations for risk stratifying more complex adnexal cysts. The O-RADS system also provides a lexicon of ultrasound descriptors to characterize, and ultimately classify, adnexal masses. For simple ovarian cysts in the premenopausal patient, the ACR recommends that 5-10 cm cysts be followed for 8-12 weeks to confirm their functional nature and to reassess for wall abnormalities. In postmenopausal females, simple cysts > 1 cm should be described but do not require follow-up imaging unless they are > 3 cm in size. The ACR O-RADS committee recommends a 1-year follow-up for 3-10 cm cysts in postmenopausal females. The complete review of this most recent SRU update and the O-RADS committee can be found in references 2 and 3.
While other guidelines in the literature are used for ultrasound evaluation of ovarian masses, the O-RADS system has been selected for review for multiple reasons. First, it provides an evidence-based standard lexicon for the use of terms that give a consistent diagnosis. Risk stratification is based upon the application of descriptors that are most predictive of malignancy from a large database, including pathology correlation with the International Ovarian Tumor Analysis (IOTA) group ultrasound rules for ovarian masses.
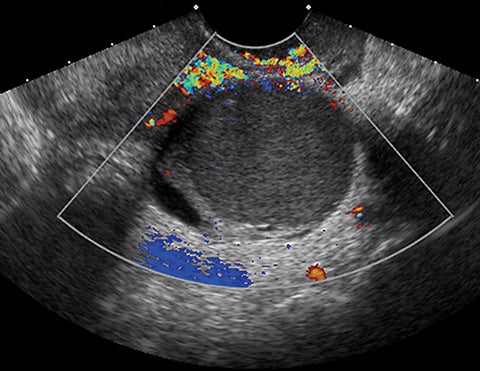
This system goes well beyond the SRU classification of simple cysts and considers other features of adnexal masses and corresponding management recommendations (Table 2). In addition, it is ultrasound-based, providing risk stratification and management based on imaging appearance of cysts. Descriptors of appearance include pure cysts, multilocular cysts, and multilocular cysts with solid components or solid masses. O-RADS considers diameter of the mass, presence of acoustic shadowing, unilocular versus multilocular cysts, cystic masses with papillary projection or solid component/solid mass appearance, and scoring of mass vascularity.3 Color flow is graded as follows:
1 – no flow;
2 – minimal flow;
3 – moderate flow; and
4 – very strong flow.
A more detailed lexicon is available in source reference 3.
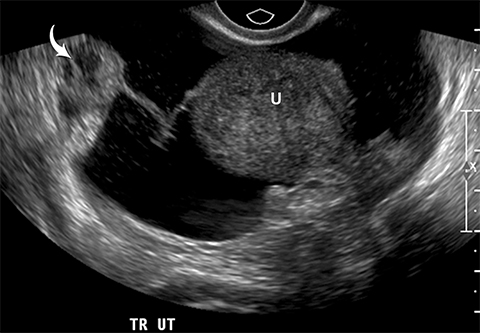
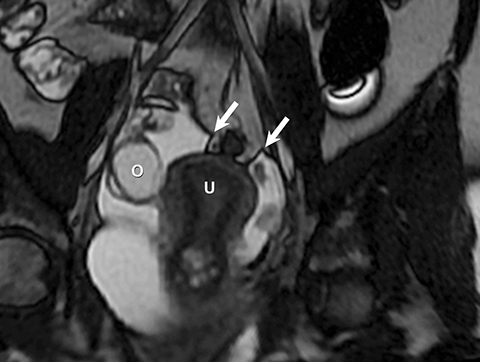
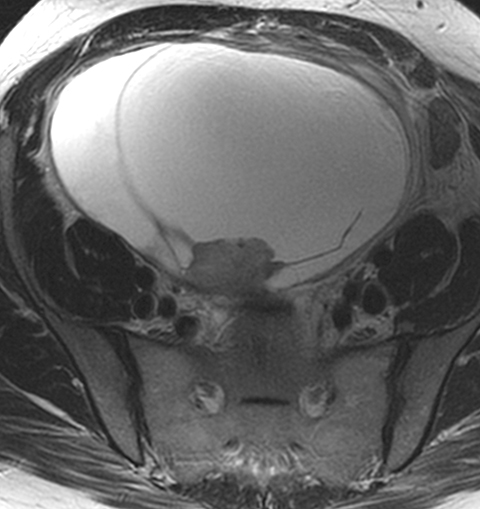
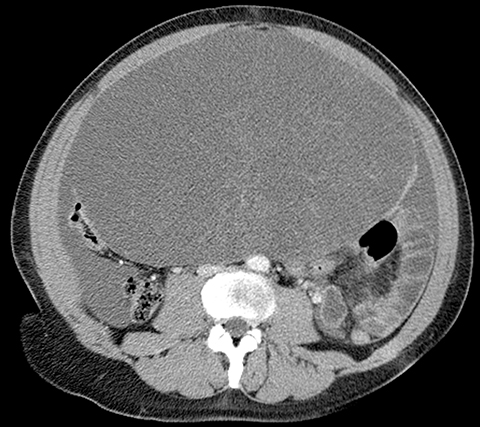
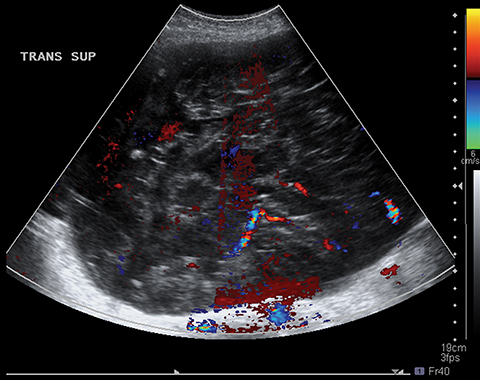
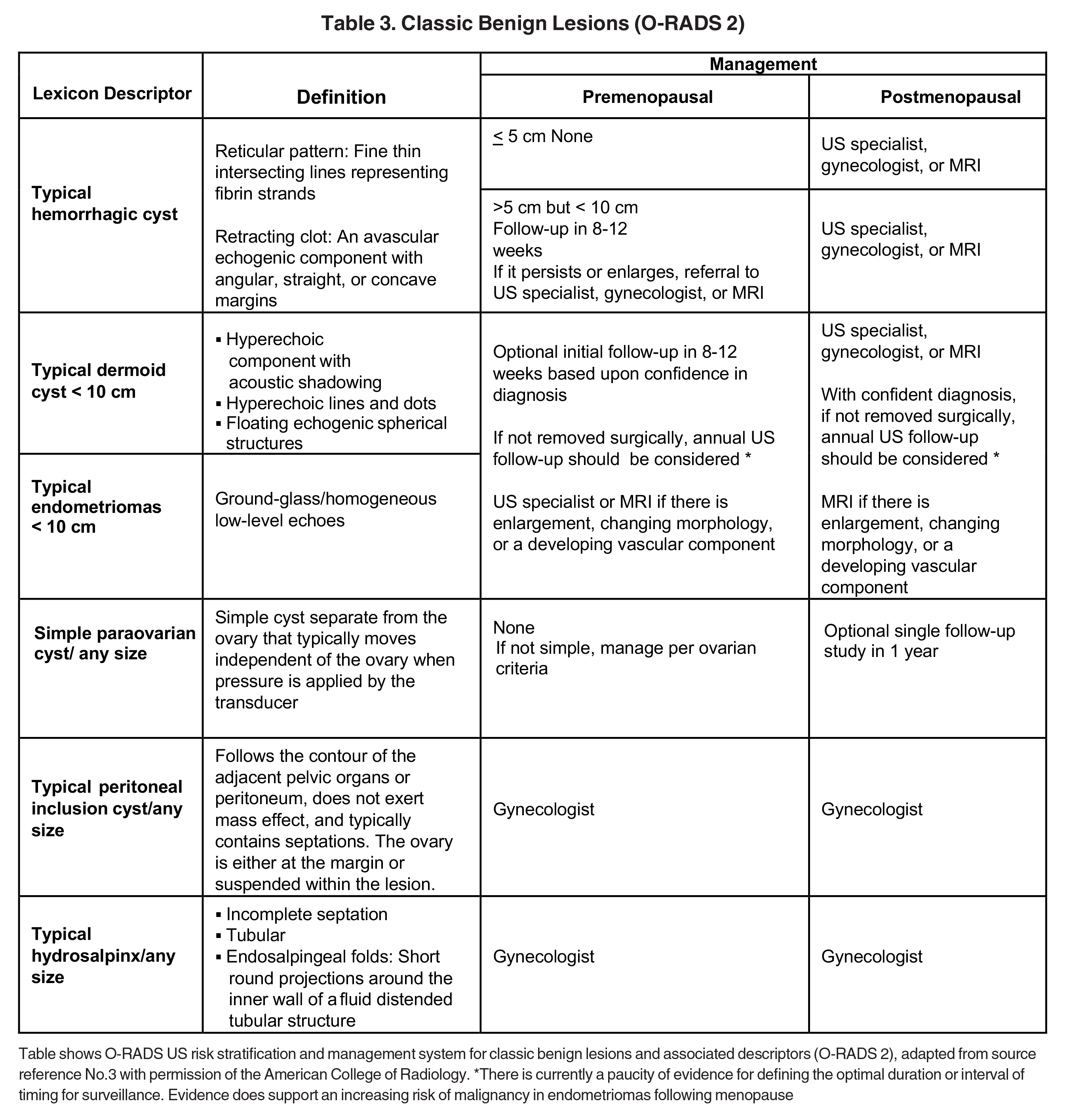
Risk stratification and management recommendations are based upon some of these parameters (Table 2). The O-RADS working group defined six categories of risk stratification: O-RADS 0, in which the adnexa are incompletely evaluated; O-RADS 1, the normal premenopausal ovary; O-RADS 2, almost certainly benign lesions with < 1% risk of malignancy; O-RADS 3, lesions with a low risk of malignancy (1-9%); O-RADS 4, lesions with an intermediate risk of malignancy (10-<50%); and O-RADS 5, high risk of malignancy (>50%).3 O-RADS 2 lesions have fairly classic features such as hemorrhagic cysts (Figure 1), dermoid cysts (Figure 2), endometriomas (Figure 3), para-ovarian cysts, peritoneal inclusion cysts (Figure 4) and hydrosalpinx (Figure 5, Table 3). These lesions will be reviewed in detail here. For O-RADS 3 or greater lesions and sometimes in post-menopausal O-RADS 2 lesions, MRI may be valuable.
Germ Cell Tumors
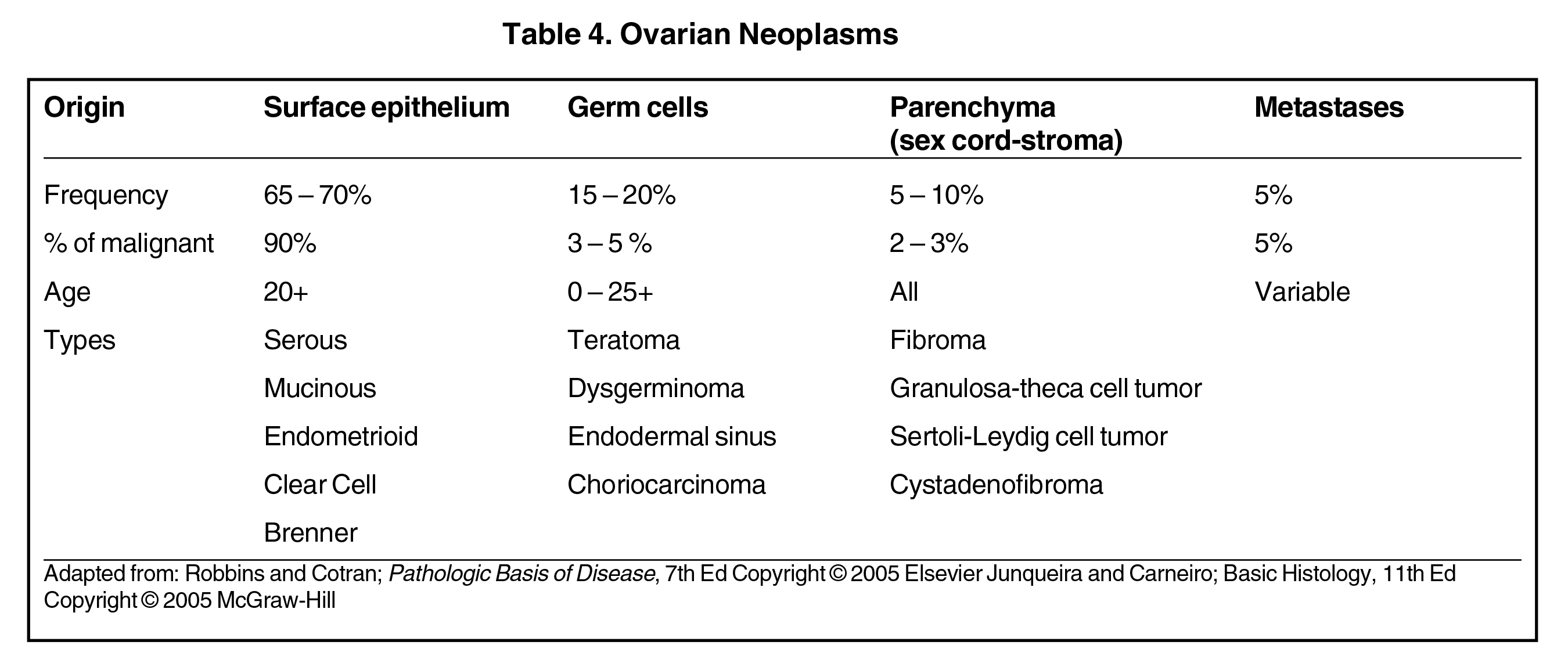
Dermoid cysts and teratomas are classified as germ cell tumors (Table 4), account for 15-20% of all ovarian neoplasms and are rarely malignant. Teratomas may be mature or immature. Dermoid cysts are mature cystic teratomas that may have components from 3 germ-cell layers that predominantly include mature ectoderm elements.
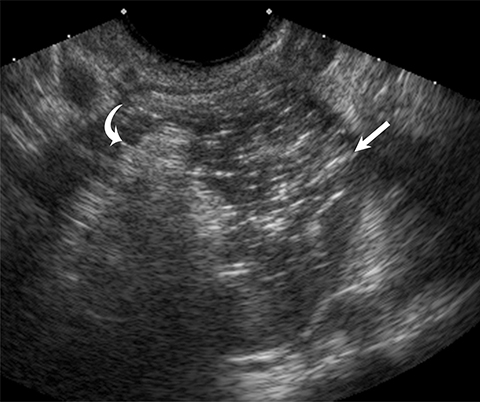
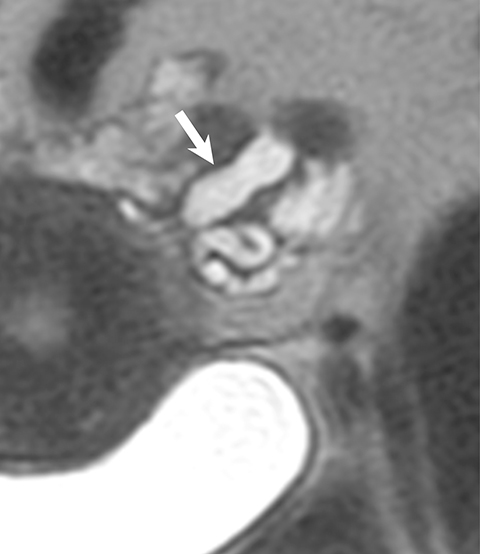
Ultrasound and CT features of dermoids include cystic components, fatty elements, hair, and/or calcifications (Figure 2). Thus, a hyperechoic component with acoustic shadowing in a predominantly cystic mass on ultrasound is highly predictive of a dermoid cyst. These cysts can also demonstrate a variety of other sonographic features, including a hyperechoic component with acoustic shadowing, hyperechoic lines and dots, or floating echogenic spherical structures. A Rokitansky nodule is a solid mass of sebaceous material projecting into the lumen of the mass. Most cases require no further evaluation upon identification of classic ultrasound or CT features of O-RADS 2 lesions.
Endometriomas
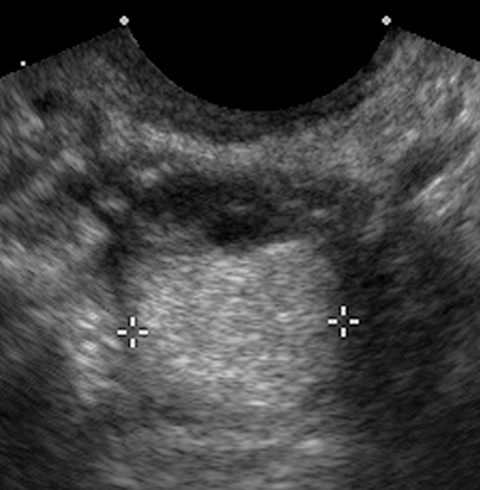
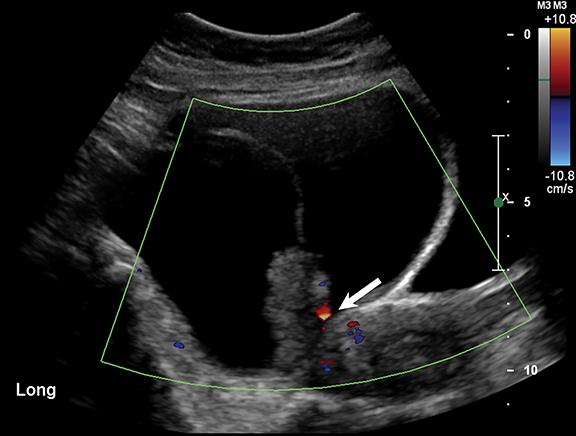
Endometriomas and hemorrhagic cysts may appear similar on sonography. However, hemorrhagic cysts may have characteristics of a retracting clot and/or reticular pattern of internal echoes (Figure 1). According to the recent O-RADS classification, masses with this characteristic pattern of a hemorrhagic ovarian cyst and < 5 cm without internal flow require no follow-up imaging. However, follow-up at 8-12 weeks is recommended for masses > 5 cm. Alternatively, endometriomas usually appear with ground glass, homogeneous, low-level, internal echoes with no solid components on ultrasound (Figure 3). Follow-up at 12 weeks is recommended in these cases.3
Peritoneal Inclusion Cysts
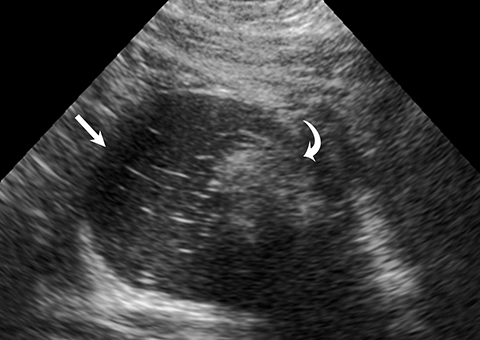
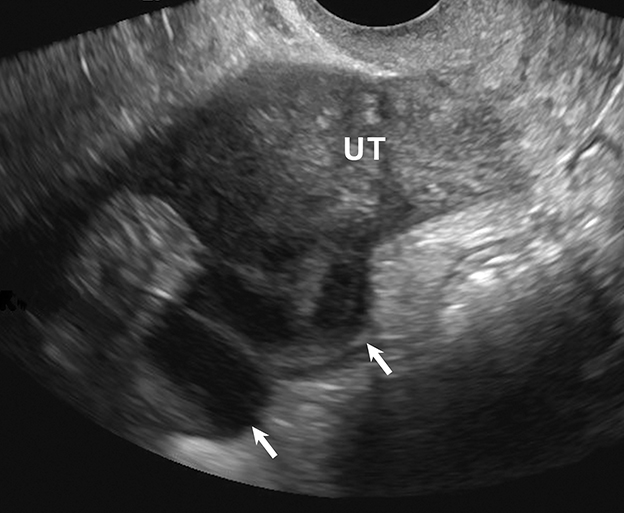
One mimic of a surface epithelial neoplasm is a benign entity called a peritoneal inclusion cyst, which may be mistaken for a complex cystic ovarian mass. Peritoneal inclusion cysts represent fluid “trapped” within the peritoneal adhesions in patients with previous history of abdominal or pelvic surgery, Crohn disease, or prior pelvic inflammatory process. These “cysts” are not spherical, but they may be oblong with more acute angulations at their margins, as the fluid is interposed between different surfaces within the pelvis (Figure 4). History is helpful in such cases. There should be no mural nodularity. They need only be evaluated with ultrasound or, if detected with CT, they require no further evaluation.
Hydrosalpinx
Diagnostic ultrasound features of hydrosalpinx include visualization of a tubular structure with septations, or an S-shaped cystic mass separate from the ovary or uterus (Figure 5). Depending on transducer angulation, this adnexal mass may appear as a more rounded cystic structure. When seen in cross-section, the longitudinal folds produce a characteristic “cogwheel” appearance.
O-RADS 4 and 5 Lesions
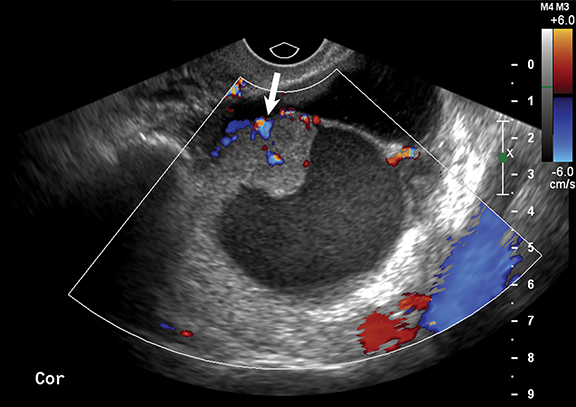
Worrisome ultrasound features of O-RADS 4 and 5 lesions include the presence of multilocular cysts, solid components within the lesion, papillary projections, large size, and higher color flow grades (Table II, Figures 6, 7). These features can be present in solid lesions such as sex cord tumors or ovarian metastases, which will be explained in greater detail in Part II of this review (Table 4). Of these lesions, the most worrisome are surface epithelial neoplasms. These tumors constitute approximately 90% of all malignant ovarian tumors and include serous or mucinous cyst adenocarcinomas. Serous neoplasms have a higher frequency of malignancy than do mucinous neoplasms.
An unusual type of surface epithelial tumor with concerning features is the borderline tumor. These tumors are typically diagnosed pathologically after surgery. They occur in younger patients and have a 10-year survival rate as high as 95% (Figure 8). Surface epithelial neoplasms will be discussed in more detail in Part II.
Conclusion
Ultrasound plays a significant role in diagnosing ovarian and adnexal pathology. It is usually the screening modality of choice in these cases and may be useful in establishing a specific diagnosis in the nonpregnant patient. The SRU and ACR O-RADSTM guidelines may be helpful in classifying simple ovarian cysts and other adnexal masses. In more problematic cases, consultation with an ultrasound expert and the addition of MRI may be warranted.
References
- Expert Panel on Women’s, Imaging, Atri M, Alabousi A, et al. ACR Appropriateness Criteria® Clinically Suspected Adnexal Mass, No Acute Symptoms. J Am Coll Radiol. 2019; 16(5S): S77-S93.
- Levine D, Patel MD, Suh-Burgmann EJ, et al. Simple adnexal cysts: SRU consensus conference update on follow-up and reporting. Radiology. 2019; 293(2): 359-371.
- ACR O-RADS for US. American College of Radiology - Ovarian-Adnexal Reporting & Data System (O-RADS). https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/O-Rads. https://www.acr.org/-/media/ACR/Files/RADS/O-RADS/O-RADS_US-Risk-Stratification-Table.pdf.
Affiliation: UC Davis Health, Sacramento, CA. Disclosures: Dr McGahan is a member of the Editorial Advisory Board of Applied Radiology.
