Pitfalls in MDCT urography
Images





Multi-detector CT (MDCT) urography has become the standard examination for unexplained, painless hematuria, allowing the assessment for numerous etiologies in a single study by imaging during multiple contrast phases. It boasts 98-100% sensitivity for urinary tract stones1 and 89-100% sensitivity for detection of urothelial carcinoma (UC).2 As a result, it has largely replaced other modalities such as ultrasound and intravenous pyelography. As with many imaging examinations, however, technical and interpretive pitfalls exist. A thorough understanding of these can help to prevent false-positive and false-negative results.
Technique
MDCT urography is tailored to visualize calculi, renal parenchyma, and the urothelium. Probably the most commonly utilized protocol is the single-bolus, or three-phase, technique. This technique involves a precontrast scan through the abdomen and pelvis with subsequent imaging during nephrographic and excretory phases after administration of IV contrast.
Multiple variations of this protocol have been developed to reduce radiation exposure and optimize imaging.3 A corticomedullary/urothelial phase may be included, contributing to a total exposure of 25-35 mSv, compared to 3.6 mSv for intravenous pyelography.4 Many choose to forgo this phase to reduce radiation exposure; others argue that its inclusion may occasionally reveal vascular lesions, such as renal AVMs or briskly enhancing urothelial tumors. Additionally, dose may be reduced on the unenhanced acquisition, as stones are easily differentiated from soft tissue even on a background of increased noise. Some have explored the use of dual-energy CT to virtually reconstruct the non-contrast portion by acquiring data with two tube potentials, although this has not yet proven reliable enough for clinical use and further refinement of this technique is ongoing.5
Another method for reducing the number of scans and radiation dose in MDCT urography is the split bolus technique, which yields a synchronous nephrographic and excretory phase by dividing the contrast volume into two boluses, separated temporally by a set delay.6 After an unenhanced abdominopelvic CT acquisition, the IV contrast dose is administered as a split dose with 75% given initially and 25% given after a 5- to 10-minute delay.
Technical pitfalls
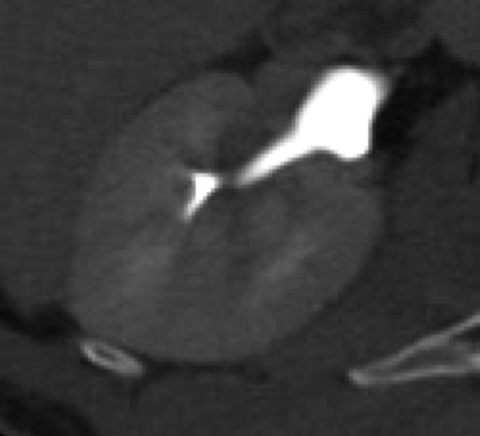
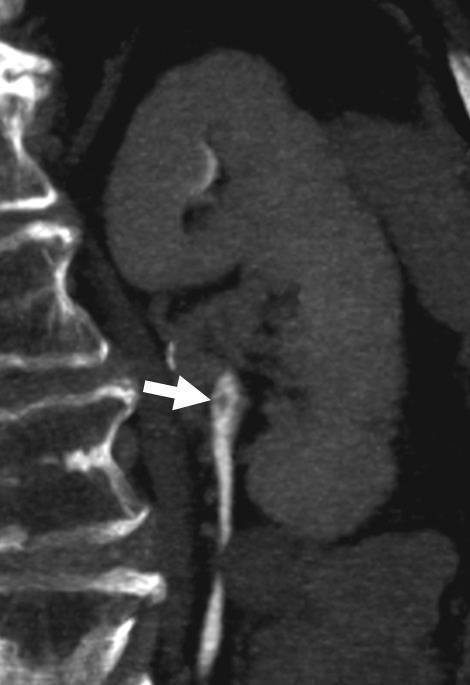
Good distention of the collecting system and ureters with excreted contrast aids in detecting urothelial tumors. A variety of techniques may improve distention. Oral hydration promotes natural diuresis and dilutes intraluminal contrast somewhat.7 This can be beneficial by decreasing streak artifact of concentrated excreted contrast (Figure 1). It is safe, easy, and inexpensive, and it doubles as a negative contrast agent in the gastrointestinal tract. Intravenous hydration has also been used and may provide better distention, but it may stimulate peristaltic contractions, as well.8, 9 Diuretics may improve mid and distal ureteral distention compared to hydration alone, as well as reducing the delay for excretory imaging due to decreased contrast attenuation within the ureters.10 Intravenous furosemide may be administered at 0.1 mg/kg, up to 10 mg, just prior to scanning after the patient has been positioned on the CT table. An important pitfall that may arise when using furosemide with a split-bolus technique is overdilution of excreted contrast material (Figure 2); a larger initial contrast bolus is required to prevent overdilution of excreted contrast material.11 At our institution, we use a 75%/25% split for the first and second bolus respectively and 5 mg furosemide prior to scanning. Finally, a theoretical pitfall exists with the use of iso-osmolar contrast agents. High-osmolality agents historically used with excretory urography resulted in a strong diuretic effect and excellent urinary tract distention. Iso-osmolar agents theoretically lack this diuretic effect and could compromise urinary tract distention but are better tolerated by patients. Low-osmolar agents may represent a good compromise of excellent safety profile, patient tolerance, and urinary tract distention.
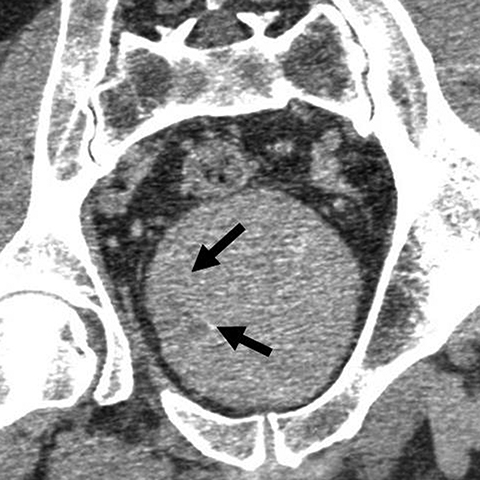
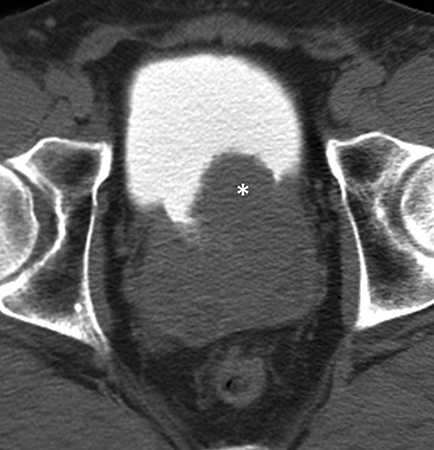
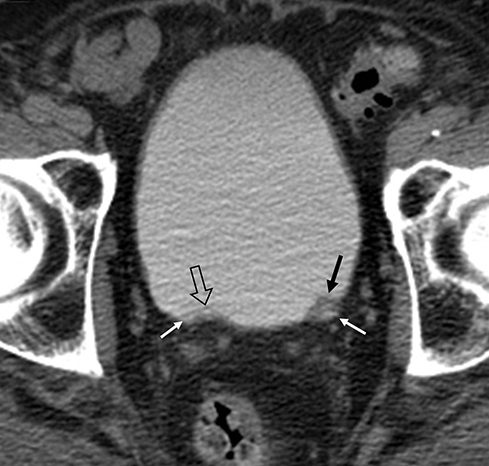
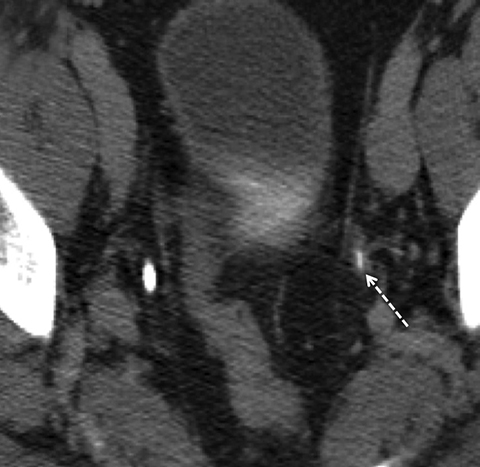
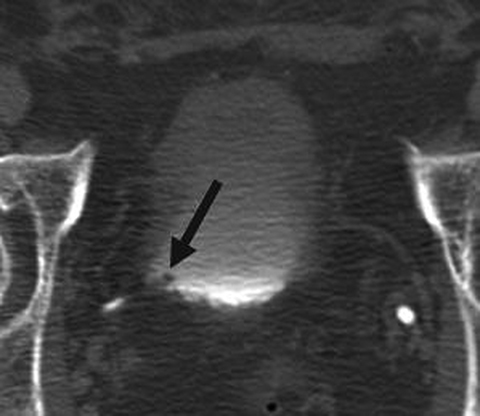
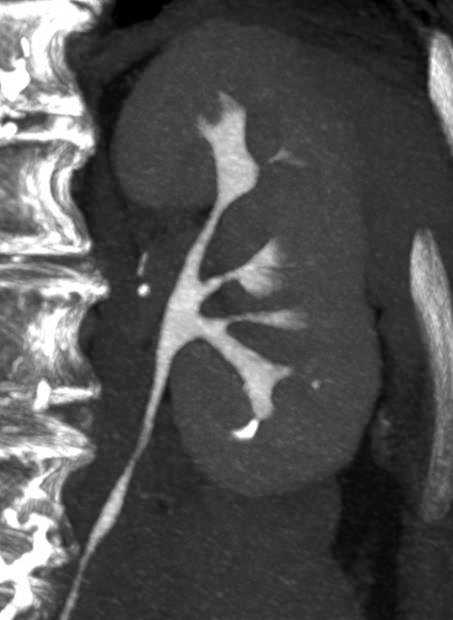
Incomplete opacification is more likely to occur in voluminous structures, such as the bladder and capacious collecting systems (Figures 3, 4), where the increased surface area of urothelium corresponds to increased likelihood of UC. Layering contrast, unopacified urine, and ureteral jets may mimic or mask disease. Moving the patient to “mix” contrast and urine in the bladder prior to pelvic scanning can result in more homogenous opacification (Figure 4). It is important to keep in mind that flat lesions, such as carcinoma in situ, may be undetectable by CT and direct visualization with cystoscopy remains the gold standard for evaluation of the bladder. Despite the shortcomings of bladder imaging by MDCT urography, a careful examination of the bladder should be conducted, as not all patients receive cystoscopy, MDCT urography may guide cystoscopy and finally, cystoscopy may occasionally miss lesions that are detectable by CT.
Interpretive pitfalls
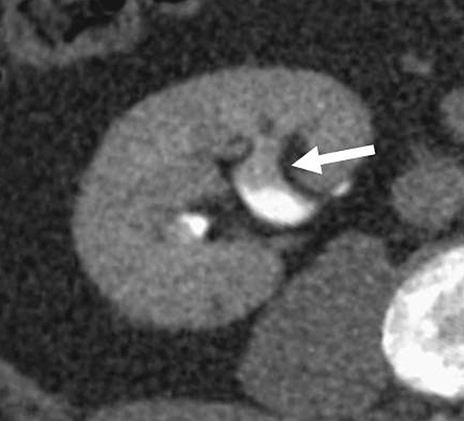
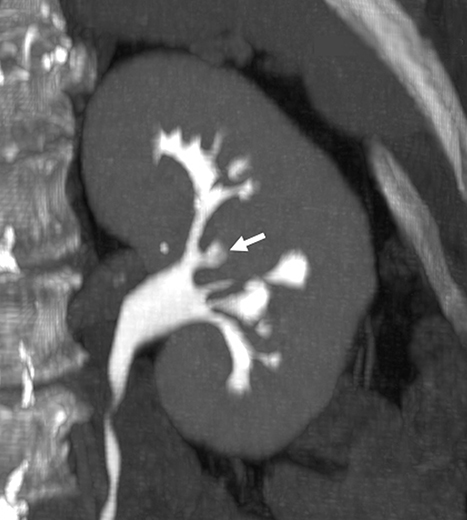
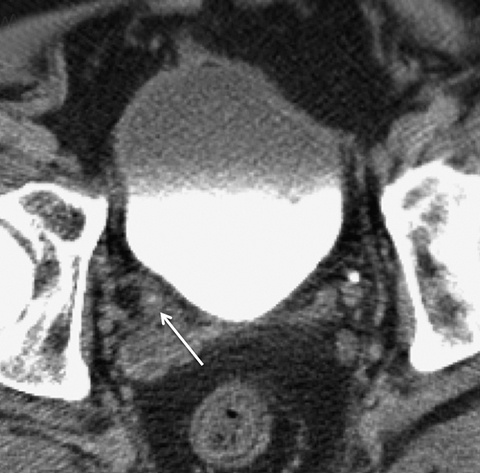
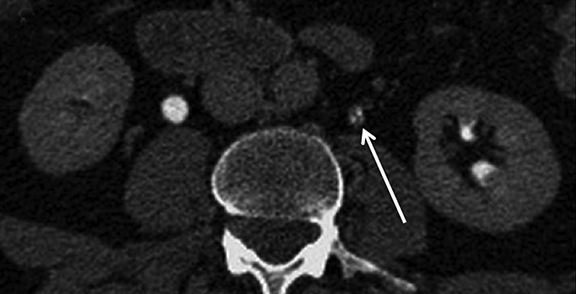
As with any imaging study, some of the most important pitfalls to be aware of are those of misinterpretation. Sometimes normal anatomic structures or variants may mimic pathology. Sometimes non-neoplastic pathologic processes may mimic malignancy. An example of a common normal anatomic structure that may mimic pathology is a prominent column of Bertin, hypertrophic normal cortical tissue extending between medullary pyramids, which may simulate a renal mass. Sometimes, a prominent column of Bertin may have its own “microcalyx” (Figure 5). Importantly, a prominent column of Bertin should follow the attenuation and imaging appearance of normal renal cortex on all pre- and postcontrast phases and should not disrupt the outer contour of the kidney.
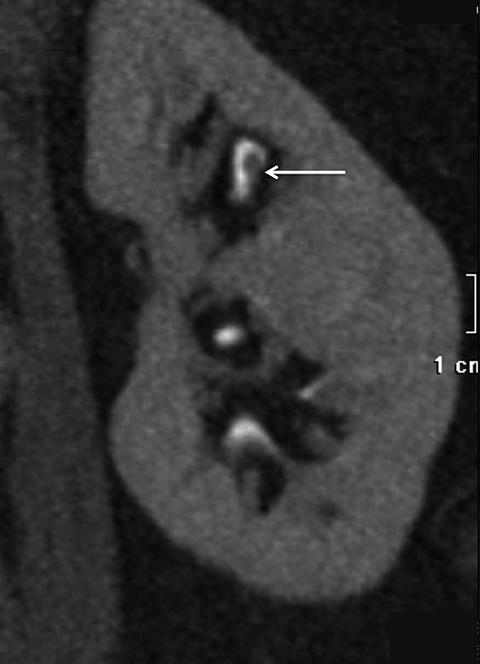
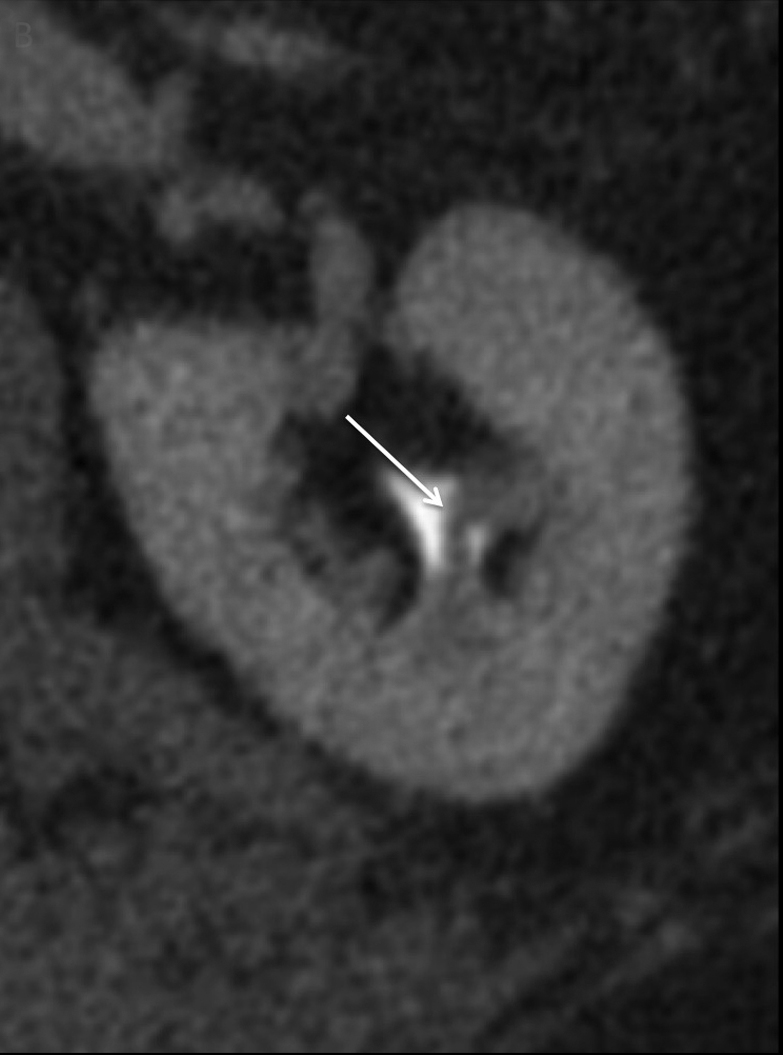
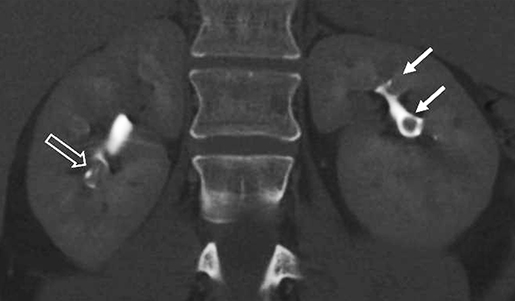
Compound papillae are a common normal variant, formed from the fusion of two simple papillae (Figure 6) and may simulate an abnormal filling defect within the renal collecting system, mimicking a urothelial neoplasm. Often patients will have multiple compound papillae. In the urinary bladder, the interureteric ridge is a normal, transversely oriented bundle of muscle fibers along the posterior wall of the bladder extending from ureteral os to ureteral os which may both mimic and mask disease as the posterior bladder is a common location for urothelial tumors (Figure 7). Familiarity with this structure, best seen when viewing in cine mode, will help to prevent misinterpretation.
A variety of non-neoplastic pathologic processes may mimic cancer, as well. MDCT urography cannot reliably distinguish urothelial thickening secondary to tumor vs inflammation, and pathologic proof may be required to confirm malignancy (Figure 8). A unique postinflammatory process in patients with chronic or recurrent urinary tract infection, ureteritis cystica, is virtually pathognomonic based on its imaging appearance of multiple, 2- to 5-mm smooth, submucosal, round, cystic projections into the urinary tract lumen (Figure 9). This process can sometimes be seen in the renal pevis (pyelitis cystica or bladder cystitis cystica). Metastatic disease (such as melanoma can sometimes simulate this appearance but is generally less smooth and homogenous than ureteritis cystica. As discussed previously, transient ureteral peristalsis may lead to nonvisualized segments of ureter as well as circumferential pseudo-thickening of the urothelium that may mimic UC. In the male urinary bladder, an enlarged, irregular prostate is a common confounding process that may either mimic or mask true disease of the bladder (Figure 4). Additionally, chronic bladder outflow obstruction, often secondary to an enlarged prostate, may result in muscular bladder trabeculations which can also mimic or mask disease (Figure 10).
Other non-neoplastic processes that may manifest as intraluminal filling defects on excretory phase images include fungus balls, blood clots, sloughed papilla in the setting of papillary necrosis (Figure 11). Any of these may cause filling defects that simulate UC. Small stones within the urinary collecting system will appear as filling defects on postcontrast images and may simulate a UC. This potentially embarrassing misinterpretation can be avoided by always correlating the abnormal filling defect with the unenhanced images to be sure it is not, in fact, a stone. The corollary to this is that not all calcifications represent stones. Urothelial carcinoma has a propensity to calcify, so it is imperative to evaluate any urinary tract calcification for an associated mass or focal urothelial thickening before calling it a urinary tract stone.
Pitfalls with reconstructed images
Reconstruction techniques such as MIP can provide useful, intuitive images and added sensitivity, as well as clarify confusing findings when used in conjunction with each other. Dillman et al demonstrated that individual reconstructions, including 1.25mm axial, 2.5mm axial, coronal MPR, and rotating CPR, all had similar sensitivities of 63-75% for detecting urothelial neoplasm. However, by using multiple techniques, sensitivity improved to 91- 94%.12 Examples of utilizing multiple reconstructions to avoid misinterpretation include the identification of kinked or tortuous ureters, which may demonstrate a bizarre appearance on axial images, simulating an intraluminal filling defect (Figure 12). Imaging during expiration rather than inspiration can help to alleviate this phenomenon.
Understanding how various reconstructions are generated is important to avoid misinterpretation. As MIP images are generated by displaying only the highest density data in a projected plane with low-density data being discarded, MIPs may obscure low-attenuation lesions, such as urothelial filling defects surrounded by dense excreted contrast in the urinary tract (Figure 14). MIPs may also create pseudolesions when neighboring high-density structures are projected into the image. This may be remedied by using a thinner slab for MIPs, which excludes undesired structures or preferably, by using multiplanar reformatted images (MPR), also known as Average Intenstiy Projection images instead of MIPs. For the reasons outlined above, MIPs and other reconstructed images should not be relied upon solely, and source images should always be interpreted to confirm findings on other series. Lastly, inappropriate window and level may obscure lesions. Window and level should be manually adjusted and will depend on the density of excreted contrast, which can vary from case to case.
Conclusion
MDCT urography is currently the preferred imaging study for evaluating painless hematuria. As with all imaging studies, a thorough understanding of the potential technical and interpretive pitfalls which exist can help to prevent misinterpretation.
References
- Fielding JR, Silverman SG, Rubin GD. Helical CT of the urinary tract. AJR Am J Roentgenol. 1999; 172(5):1199–1206.
- Tsili AC, Efremidis SC, Kalef-Ezra J, et al. Multidetector row CT urography on a 16-row CT scanner in the evaluation of urothelial tumors. Eur Radiol. 2007; 17(4):1046–1054.
- Silverman SG, Leyendecker JR, Amis ES Jr. What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology. 2009; 250(2):309–323.
- Caoili EM, Inampudi P, Cohan RH, et al. Optimization of multi-detector row CT urography: effect of compression, saline administration, and prolongation of acquisition delay. Radiology. 2005; 235(1):116–123.
- Graser A, Johnson TR, Chandarana H, et al. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol. 2009; 19(1):13–23.
- Chow LC, Kwan SW, Olcott EW, et al, Split-bolus MDCT urography with synchronous nephrographic and excretory phase enhancement. AJR Am J Roentgenol. 2007; 189(2):314-322.
- Kawamoto S, Horton KM, Fishman EK. Opacification of the collecting system and ureters on excretory-phase CT using oral water as contrast medium. AJR Am J Roentgenol. 2006; 186(1):136–140.
- Sanyal R, Deshmukh A, Sheorain V, et al. Urography: a comparison of strategies of upper urinary tract opacification. Eur Radiol. 2006;17(5):1262–1266
- Sudakoff GS, Dunn DP, Hellman RS, et al. Opacification of the genitourinary collecting system during MDCT urography with enhanced CT digital radiography: nonsaline versus saline bolus. AJR Am J Roentgenol. 2006; 186(1):122–129
- Silverman SG, Akbar SA, Mortele KJ, et al. Multi-detector row CT urography of normal urinary collecting system: furosemide versus saline as adjunct to contrast medium. Radiology . 2006;240(3):749–755.
- Saket R, Chow LC, Schraedley-Desmond P, et al. Optimizing split-bolus CT urography. Society of Uroradiology Annual Meeting, March 2004, Scottsdale, AZ
- Dillman JR, Caoili EM, Cohan RH, et al. Detection of upper tract urothelial neoplasms: sensitivity of axial, coronal reformatted, and curved-planar reformatted image-types utilizing 16-row multi-detector CT urography. Abdom Imaging. 2008;33(6):707-716.
Citation
R M, H H, L C. Pitfalls in MDCT urography. Appl Radiol. 2018;(12):16-21.
December 3, 2018