Sclerosing adenosis mimicking malignant lesion on breast MRI
Images


CASE SUMMARY
A 45-year-old female presented with an abnormal screening mammogram, and subsequent diagnostic mammogram and ultrasound appearance were nonconclusive. MRI was recommended for further evaluation and showed a Type 3 enhancing kinetic curve highly sug- gestive of malignancy. Therefore, core biopsy was performed and revealed sclerosing adenosis. Diagnosis of sclerosing adenosis can be difficult with mammogram, ultrasound, and MRI, and tissue diagnosis may be required for definitive diagnosis. There have only been a few cases in the literature reporting sclerosing adenosis presenting with classic features of malignancy, ultimately diagnosed as benign scleros- ing adenosis.
IMAGING FINDINGS
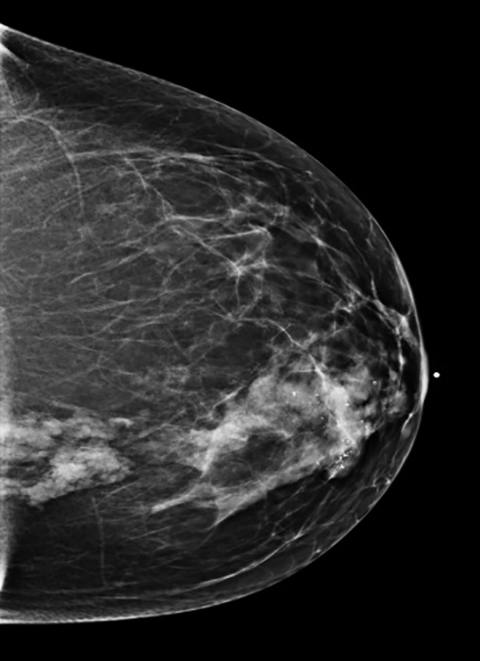
A 45-year-old Caucasian female presented with a history of right breast biopsy in 2011 that proved to be benign. Her annual screening mam- mogram continued to demonstrate a focal asymmetry within the central medial right breast as well as a group of coarse calcifications in the retroareolar region surrounding the biopsy clip. In 2013, her annual screening mammogram revealed that the focal asymmetry in the right breast was unchanged in size but had increased in density, and the associated calcifications had increased in number (Figure 1). Therefore, a contrast- enhanced MRI breast was recommended.
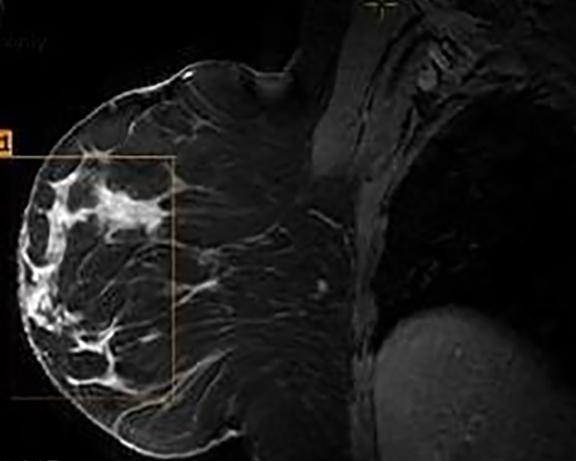
The MRI revealed a large area of abnormal enhancement within an area of asymmetric glandular tissue (Figure 2), which corresponded with the focal asymmetry seen on the mammogram. The enhancement pattern of this area included a mixture of Type 1, Type 2, and Type 3 washout curves. The areas of abnormal enhancement had been sampled in 2011, and at that time, pathology results had revealed sclerosing adenosis.
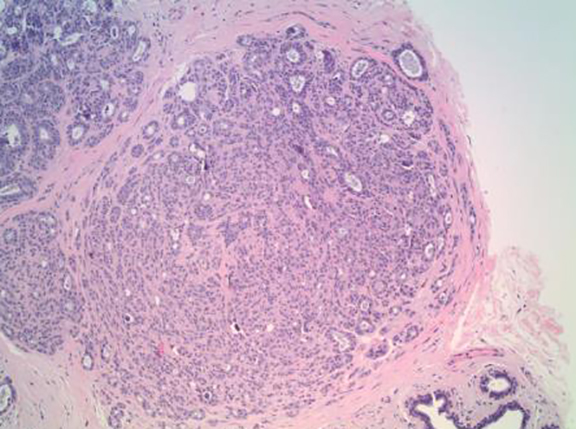
Although there had been no significant change in the overall size or the enhancement of this area since the prior mammogram and MRI in 2011, the asymmetric abnormality seen on mammogram and MRI was atypical for sclerosing adenosis. It was classified as BI-RADS 4 (suspicious finding), and repeat MRI-guided biopsy of the abnormal enhancing area was performed. Histopathology again revealed benign breast tissue, including sclerosing adenosis (Figures 3 and 4), ductal hyperplasia of the usual type, duct ectasia, small benign papilloma, and fibrocystic changes. Microcalcifications associated with benign ducts were also present in the specimen.
DIAGNOSIS
Atypical sclerosing adenosis
DISCUSSION
Sclerosing adenosis is a proliferative lesion of the terminal duct lobular unit consisting of disordered and increased number of acini. It is a known mimic of malignancy as it may produce suspicious diffuse or focal calcifications, and it may also produce a palpable mass, in which case it is known as “florid adenosis” or “adenosis tumor”.1-4
Its etiology is unknown. Given that is seen mostly in perimenopausal women and occasionally regresses after menopause, there is some speculation that it results from estrogen stimulating proliferation of epithelial tissue in the breast. Sclerosing adenosis is usually asymptomatic and is often an incidental microscopic finding in breast tissue removed for another indication. It may present clinically with mastalgia, and the nodular form can present as a palpable mass.5,6
The main histopathologic finding of sclerosing adenosis is alteration of the terminal ductal lobular unit presenting as a widening, swirling, and distortion of the lobules with numerous crowded acini in the early stages and stromal fibrosis in the later stages. The gross specimens and microscopy evaluation can prove challenging. Sclerosing adenosis can appear in a lobulocentric pattern and may be mistaken for tubular carcinoma.7,8 It may also present with an infiltrative pattern and may be confused with invasive lobular carcinoma.7,9
Sclerosing adenosis is part of the spectrum of fibrocystic changes in the breast, and it is strongly associated with various proliferative lesions, including epithelial hyperplasia, intraductal papilloma, complex sclerosing lesions, and apocrine changes. Sclerosing adenosis can also coexist with both invasive and in situ cancers, lobular more frequent than ductal type.2,5,10,11 Furthermore, some studies have found sclerosing adenosis to be a risk factor for invasive breast cancer apart from its association with other proliferative lesions of the breast.2,3,10-12
Mammographically, sclerosing adenosis may present as a focal asymmetry with associated clustered, round, or punctate microcalcifications. It can occasionally present as architectural distortion, irregular density, or a spiculated mass. The nodular form may appear as a well circumscribed oval or lobular mass. The combination of these features, therefore, can mimic breast carcinoma on mammography.7
Its sonographic appearance is also nonspecific, including oval hypoechoic nonshadowing masses with circumscribed, indistinct, or microlobulated margins. It may appear solid or complex cystic. On ultrasound, nodular adenosis cannot be differentiated from other benign processes such as fibroadenoma, or from well circumscribed malignancy, such as medullary carcinoma.7
Sclerosing adenosis is one of the few benign breast lesions which can demonstrate a Type 3 enhancement or kinetic curve on MRI. The MRI enhancement may be patchy or more diffuse and depends on its histologic pattern. Sclerosing adenosis may present as ductal enhancement or as a homogeneously enhancing mass that is oval or round with lobulated or angular margins. It usually has an intermediate signal intensity on T1- and T2- weighted images, with either Type 2 or Type 3 curves. While sclerosing adenosis can demonstrate Type 1 curves, many cases show Type 2 or Type 3 curves, and biopsy may be necessary to make the final diagnosis.7,9
In general, there are three types of MRI enhancement or kinetic curves. Type 1 curves have a slow and continued rise with time and are associated with benign lesions, although they have a 6-9 percent chance of being malignant. The Type 3 curve shows a rapid initial rise, followed by a drop-off with time (washout) in the delayed phase. A lesion with Type 3 kinetics is strongly suggestive of malignancy (29-77 percent). The Type 2 curve has an initial rise followed by a plateau in the delayed phase. The likelihood of a lesion with Type 2 kinetics being malignant is intermediate, between the 6 percent of the Type 1 curve and the 29-77 percent of the Type 3 curve. Many physicians will consider biopsy of lesions with Type 2 kinetics depending on lesion morphology, and most will recommend biopsy of lesions with Type 3 kinetics given the high association with malignancy.9,13
The suspicious enhancing lesion in our patient had both Type 2 and Type 3 enhancing curves; therefore, it was biopsied. Although this patient’s breast lesion had been biopsied in the past and previously had revealed benign sclerosing adenosis, since it continued to show suspicious features on mammogram, ultrasound, and MRI enhancement kinetic analysis, a repeat biopsy was performed to confirm its benign nature.
CONCLUSION
Sclerosing adenosis is a known mimic of malignancy when it presents either as suspicious calcifications or as a mass. It is strongly associated with various proliferative lesions, including epithelial hyperplasia, intraductal or sclerosing papilloma, complex sclerosing lesion, calcification, and apocrine changes. To a lesser extent, if may be associated with invasive and in situ cancers. The hypervascularity and washout kinetics of sclerosing adenosis make it extremely difficult to distinguish radiologically from carcinoma, and biopsy may be necessary for final diagnosis.
Furthermore, sclerosing adenosis may continue to show suspicious features at imaging after biopsy, raising the consideration of disconcordant results. Sclerosing adenosis has been shown to be present in 12% of benign and 5% to 7%of malignant specimens on histopathological examination.2,4,8,14 Despite its benign nature, since some studies have reported a rare association with malignant lesions, clinicians could consider resecting it at initial presentation so that it does not continue to be a dilemma for the clinician, the radiologist, and the patient.
REFERENCES
- Guray M, Sahin Benign breast diseases: Classification, diagnosis, and management. The Oncologist. 2006;11(5):435-449.
- Taskin F, Koseoglu K, et Sclerosing adenosis of the breast: Radiologic appearance and efficiency of core needle biopsy. Diagn Interv Radiol. 2011;17:131-316.
- MacErlean D, Nathan Calcifications in sclerosing adenosis simulating malignant breast calcification. Br J Radiol. 1972;45:944–945.
- Tavassoli Pathology of the breast. Norwalk: Appleton and Lange. 1992;93-97.
- DiPiro P, Gulizia J, Lester S, Meyer Mammographic and sonographic appearances of nodular adenosis. Am J Roentgenol. 2000;175:31-34.
- Gunhan-Bilgen I, Memis A, et Sclerosing adenosis: Mammographic and ultrasonographic findings with clinical and histopathological correlation. Euro J Radiol. 2002;44:232- 238.
- Elizabeth M, Liberman Breast MRI: Diagnosis and intervention. New York, NY: Springer, 2005.
- Visscher D, Nassar A, Degnim A, Frost M, et Sclerosing adenosis and risk of breast cancer. Breast Cancer Res Treat. 2014;144:205-212.
- Molleran VM, Mahoney Breast MRI. Philadelphia: Elsevier-Saunders; 2014.
- Nielsen NS, Nielsen Mammographic features of sclerosing adenosis presenting as a tumor. Clin Radiol. 1986;37:371–373.
- Preece Sclerosing adenosis. World J. Surg. 1989;13:721–725.
- Gill H, Bluffe O, Berg When is a diagnosis of sclerosing adenosis acceptable at core biopsy? Radiology. 2003(228);50-57.
- Macura K, Ouwerkerk R, Jacobs M, Bluemke Patterns of enhancement of breast MRI images: Interpretation and imaging pitfalls. Radiographics. 2006;26(6):1719-1735.
- Jensen R, Page D, Dupont W, Rogers L. Invasive breast cancer risk in women with sclerosing adenosis. Cancer. 1989;64(10):1977-83.
Citation
E P, M C, T B, H KZCRAE. Sclerosing adenosis mimicking malignant lesion on breast MRI. Appl Radiol. 2016;(4):28-31.
April 4, 2016