Thoracic Outlet Syndrome: Review of Surgical Approaches and Radiographic Complications
By Hemp J, McGriff E, Kern J, Olazagasti J, Cherry K, Hanley M
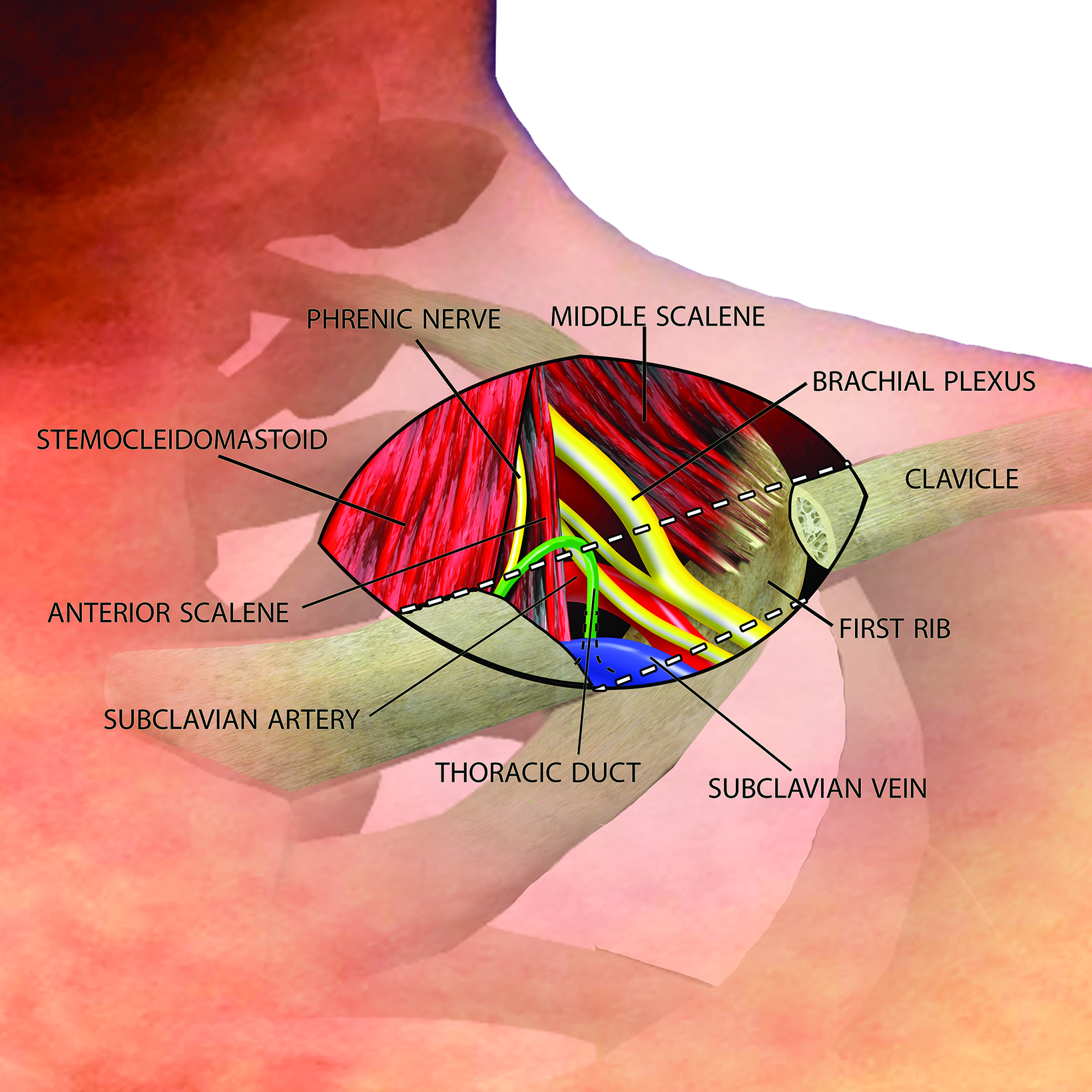
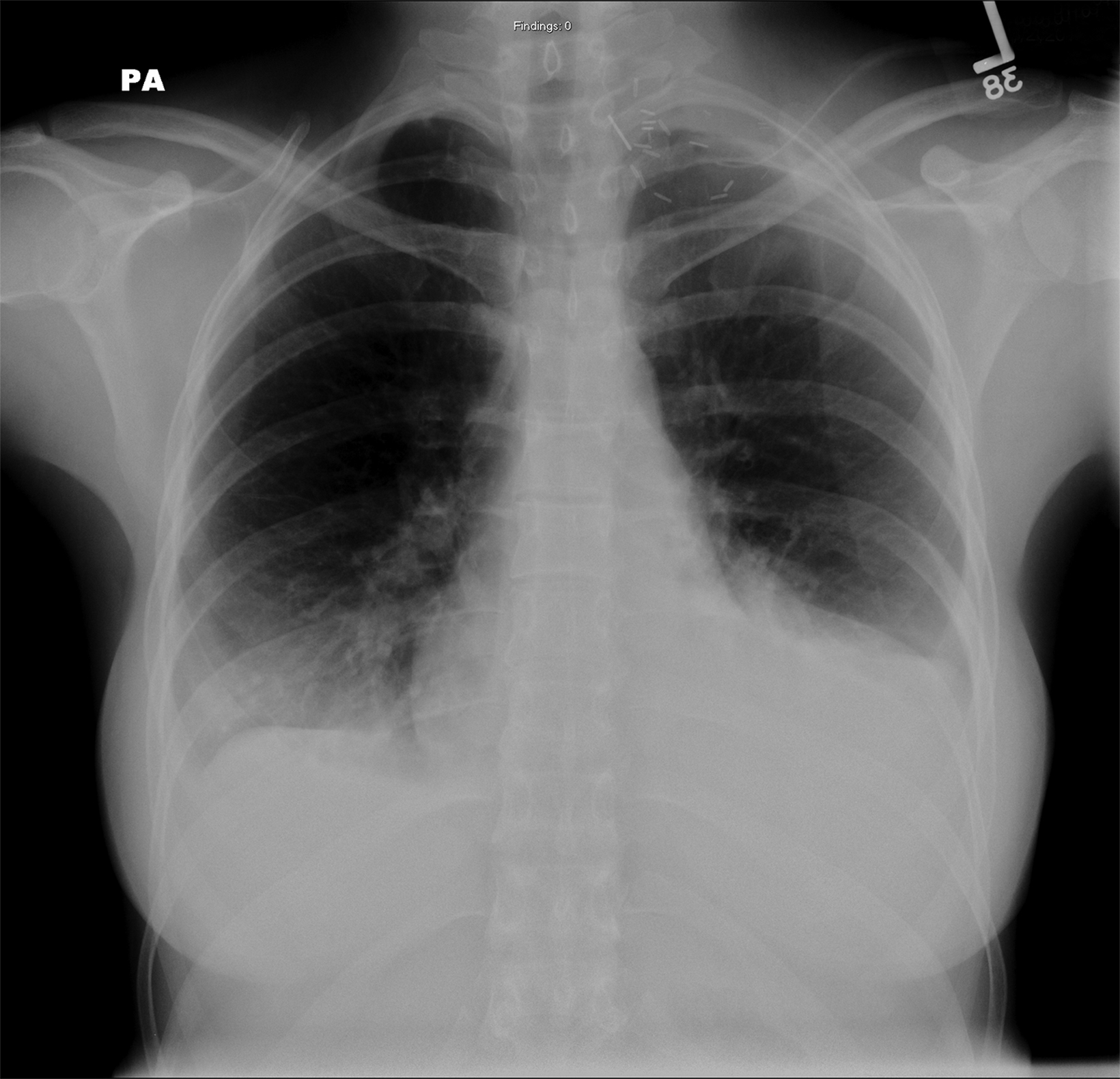
The thoracic outlet extends medially from the edge of the first rib to the mediastinum, with the clavicle forming the roof and the superior surface of the first rib forming the floor (Figure 1).1
The brachial plexus and subclavian vessels pass over the first rib in the costoclavicular space, with the brachial plexus and subclavian artery located between the anterior and middle scalene muscles in the interscalene triangle and the subclavian vein located anterior to the anterior scalene muscle between the costoclavicular ligament and subclavius muscle.
Thoracic outlet syndrome (TOS) is the clinical syndrome that results when any one of these three neurovascular structures are compressed in the already compact thoracic outlet.
The incidence of TOS has been estimated at 5:100,000.2 There are three subtypes of TOS: neurogenic, arterial, and venous. Neurogenic TOS accounts for approximately 95% of cases.3 Presenting symptoms include pain, paresthesias, numbness, and weakness in the arm and hand, with symptoms occurring predominantly along the ulnar nerve distribution in 90% of patients.4 Distinguish- ing symptoms of this subtype include pain in the shoulder and neck as well as occipital headaches.5 Although magnetic resonance imaging (MRI) may show inflammation along the brachial plexus, neurogenic TOS is a clinical diagnosis of exclusion. The majority of patients respond to conservative therapy, with thoracic outlet decompression (TOD) surgery reserved for patients with refractory disabling symptoms.
Arterial TOS is rare, accounting for 1-2% of cases.3 This subtype is usually associated with a cervical rib or anomalous first rib.5 Compression of the subclavian artery leads to stenosis and poststenotic aneurysmal dilatation, often with associated thrombus formation. Patients may present with symptoms ranging from pain in the form of claudication to critical limb ischemia from distal throm- boembolization.
Patients in the latter group require emergent surgery, including embolectomy. Ultrasound or angiography confirms the diagnosis, and a CT angiogram is essential if arterial reconstruction is planned during TOD.
Venous TOS accounts for approximately 3-5% of cases.3 Patients may present with fixed or positional pain, edema, and cyanosis in the affected extremity, with distended superficial veins and a prominent venous collateral network.5 Frequently, patients present urgently with acute thrombosis of the axillosubclavian vein, known as Paget-Schroetter syndrome, and often undergo venolysis prior to TOD, which itself carries a risk of bleeding. Ultrasound or venography confirms the diagnosis of venous TOS.
Surgical decompression of the thoracic outlet typically includes at least partial first rib resection with anterior scalenectomy.3 Depending on the indication, different parts of the rib will be taken. Resection of cervical ribs and abnormal fibrous bands is routine.
The interscalene triangle can be enlarged with middle scalenotomy and brachial plexus neurolysis in cases of neurogenic or arterial TOS, and the costoclavicular space can be further widened with resection of the costoclavicular ligament and subclavius muscle in cases of venous TOS.
Nearly 90% of patients report excellent or good functional outcomes after surgical decompression; however, a history of trauma decreases the chances of a good result.6 A symptom recurrence rate of 5-10% has been reported.7 Major complications of TOD surgery include nerve injury (Horner syndrome, brachial plexus, phrenic nerve, or long thoracic nerve), pneumothorax, pleural effusion, chylothorax, hemothorax, or extrapleural hematoma.
Many of these complications are detectable on routine postoperative plain radiograph. Thus, radiologists play an important role in the postoperative evaluation of these patients, providing an early opportunity for detection of complications and communication with the surgical team.
The purpose of this review is to discuss operative techniques of TOD along with relevant thoracic anatomy, to present examples of radiographically detectable complications, and to correlate these complications with their clinical management.
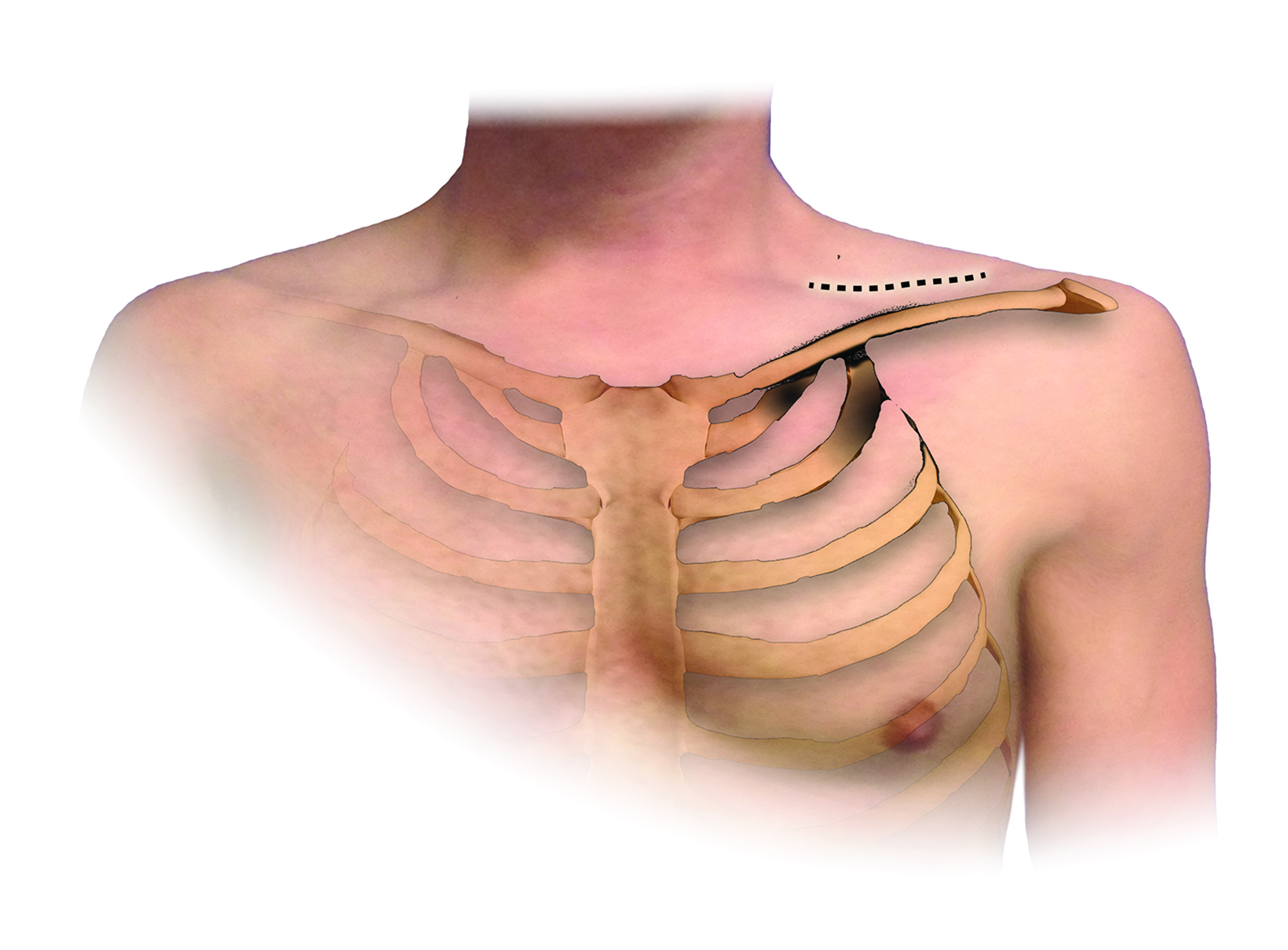
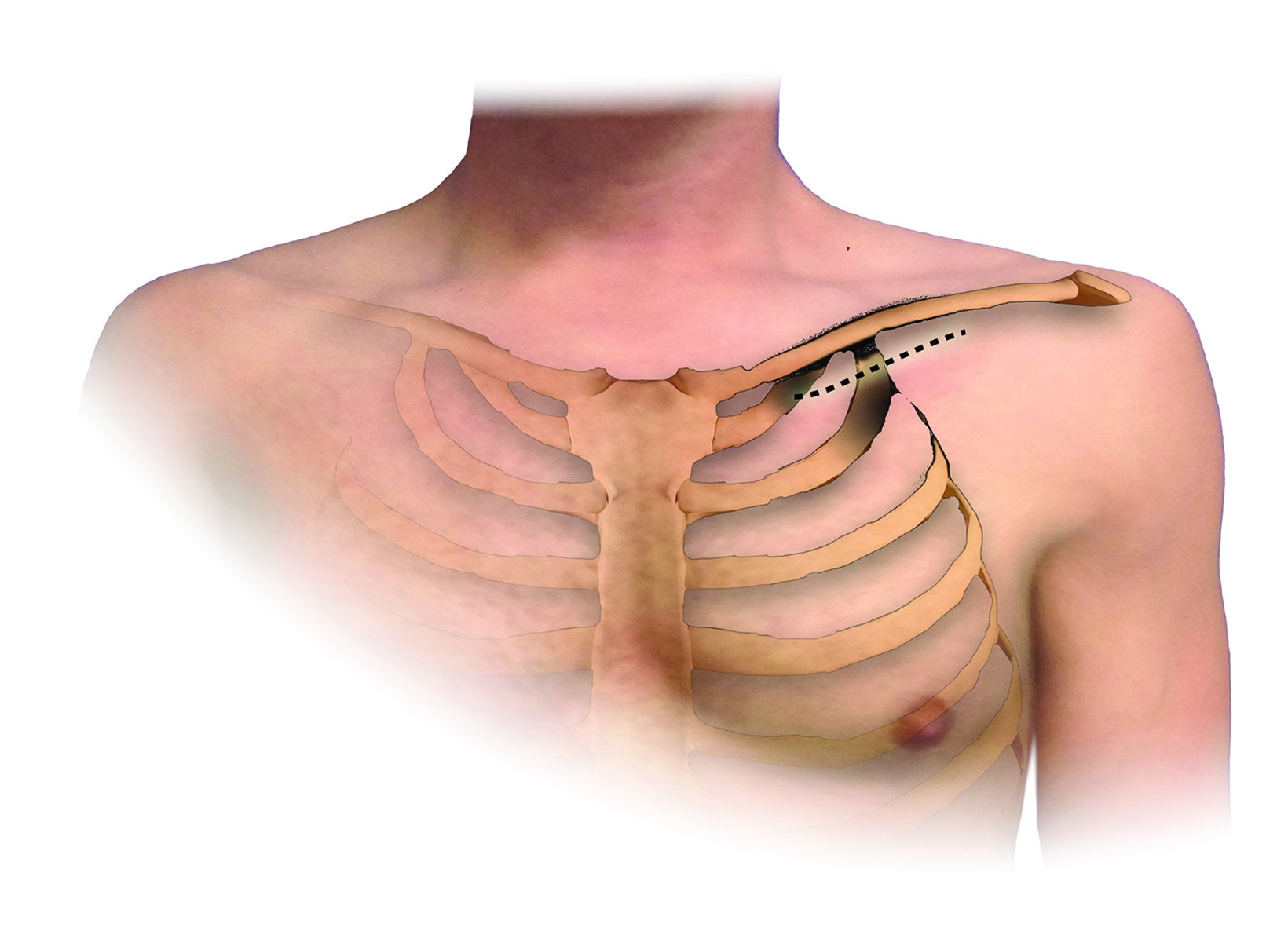
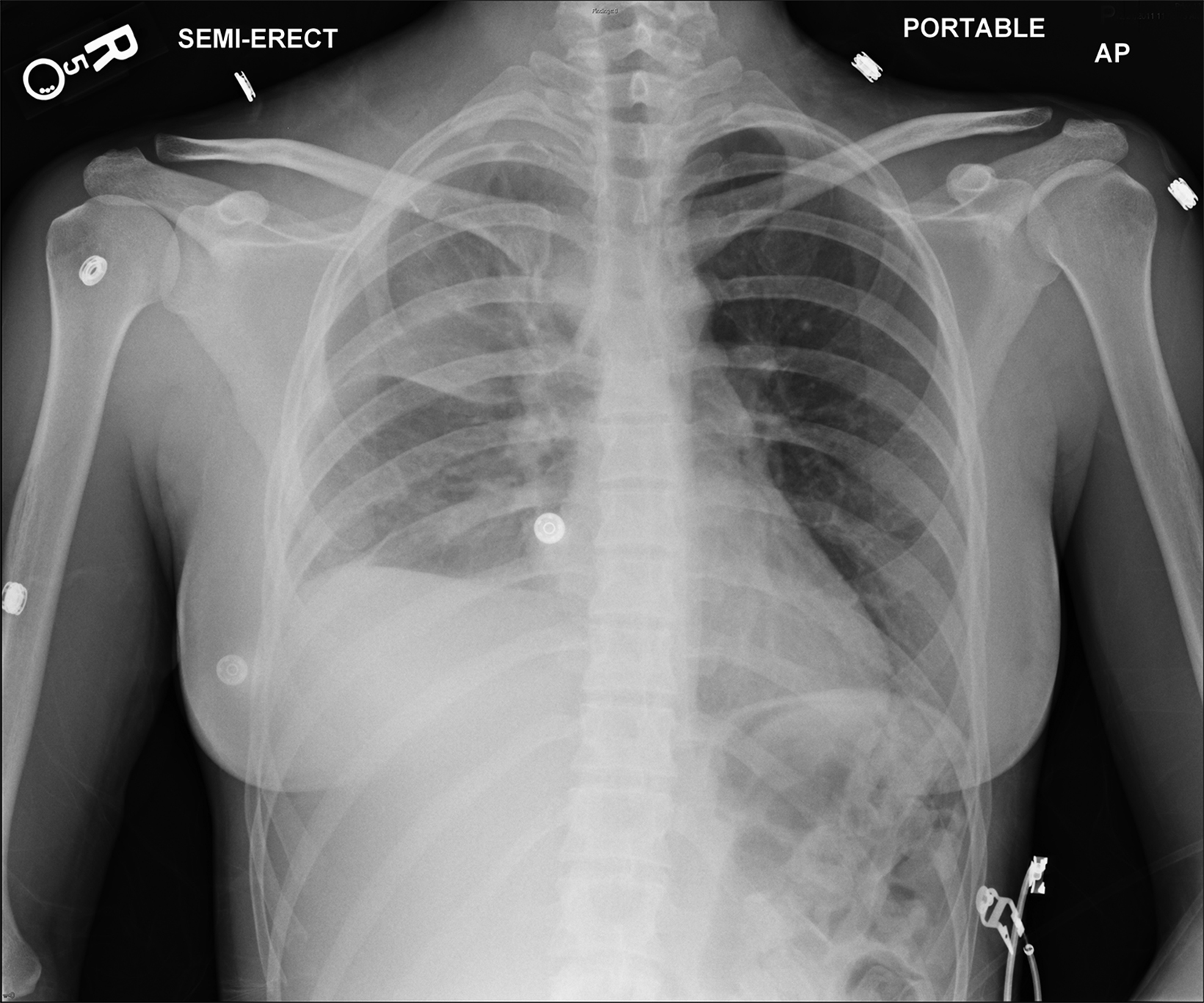
An understanding of the basic anatomy (Figure 1) and surgical techniques in the treatment of TOS is necessary to anticipate the possible complications detectable on routine postoperative chest radiographs after thoracic outlet decompression. At our institution, decompression for neurogenic or arterial TOS consists of first-rib resection, anterior scalenectomy, middle scalenotomy, and brachial plexus neurolysis through a supraclavicular approach, while venous TOS is treated with first-rib resection, anterior and middle scalenotomy, and resection of the subclavius muscle and costoclavicular ligament through an infraclavicular approach (Figure 2). Clinical and radiographic presentation of each identified surgical complication is reviewed.
Phrenic Nerve Injury
The phrenic nerve courses inferomedially along the anterior surface of the anterior scalene muscle and may be injured during dissection, retraction, or anterior scalenectomy. The injury can range from a temporary neuropraxia after stretch to a permanent deficit due to transection. Clinically, phrenic nerve injury results in paralysis of the ipsilateral hemidiaphragm with a decrease in tidal volume.
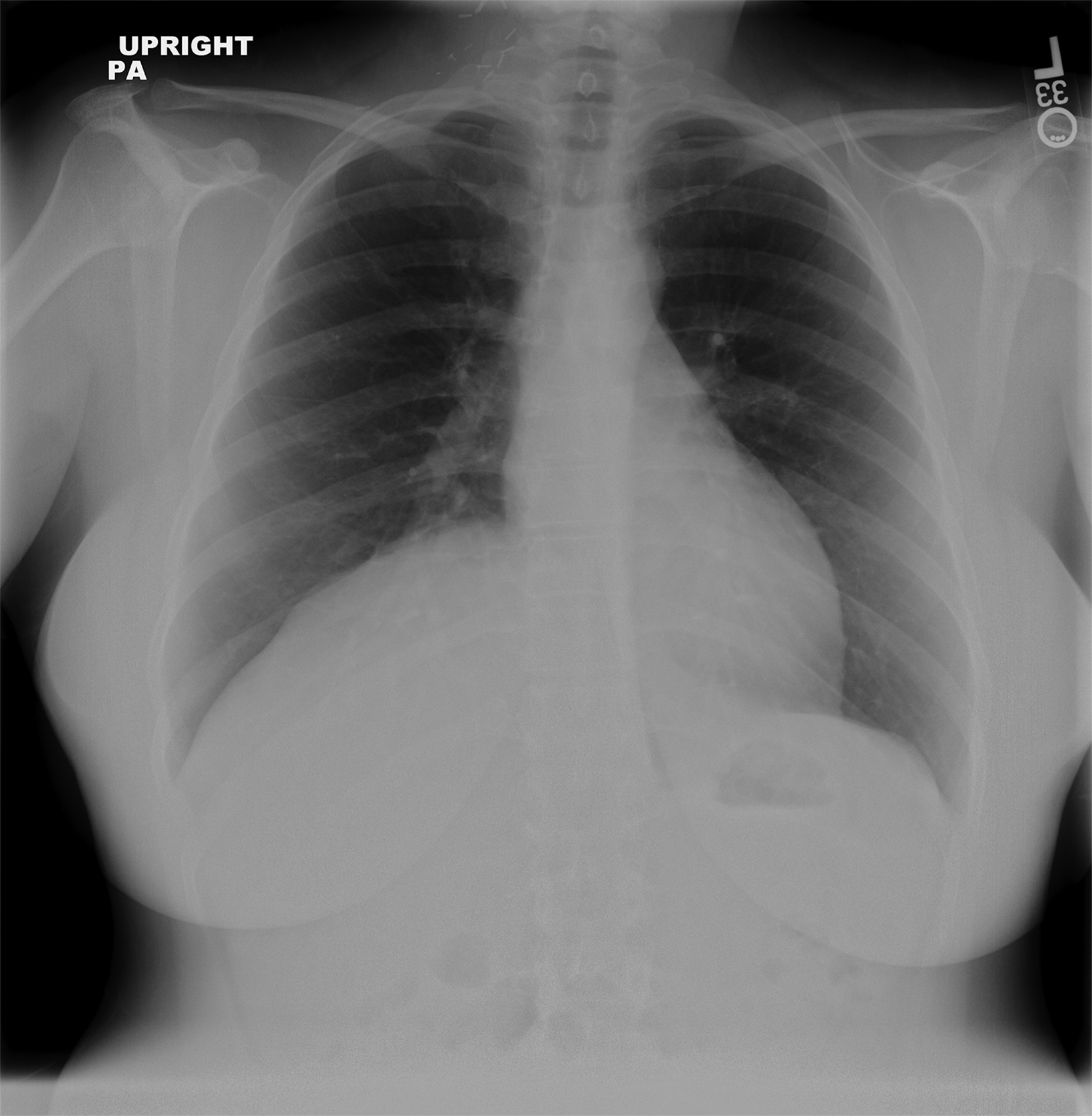
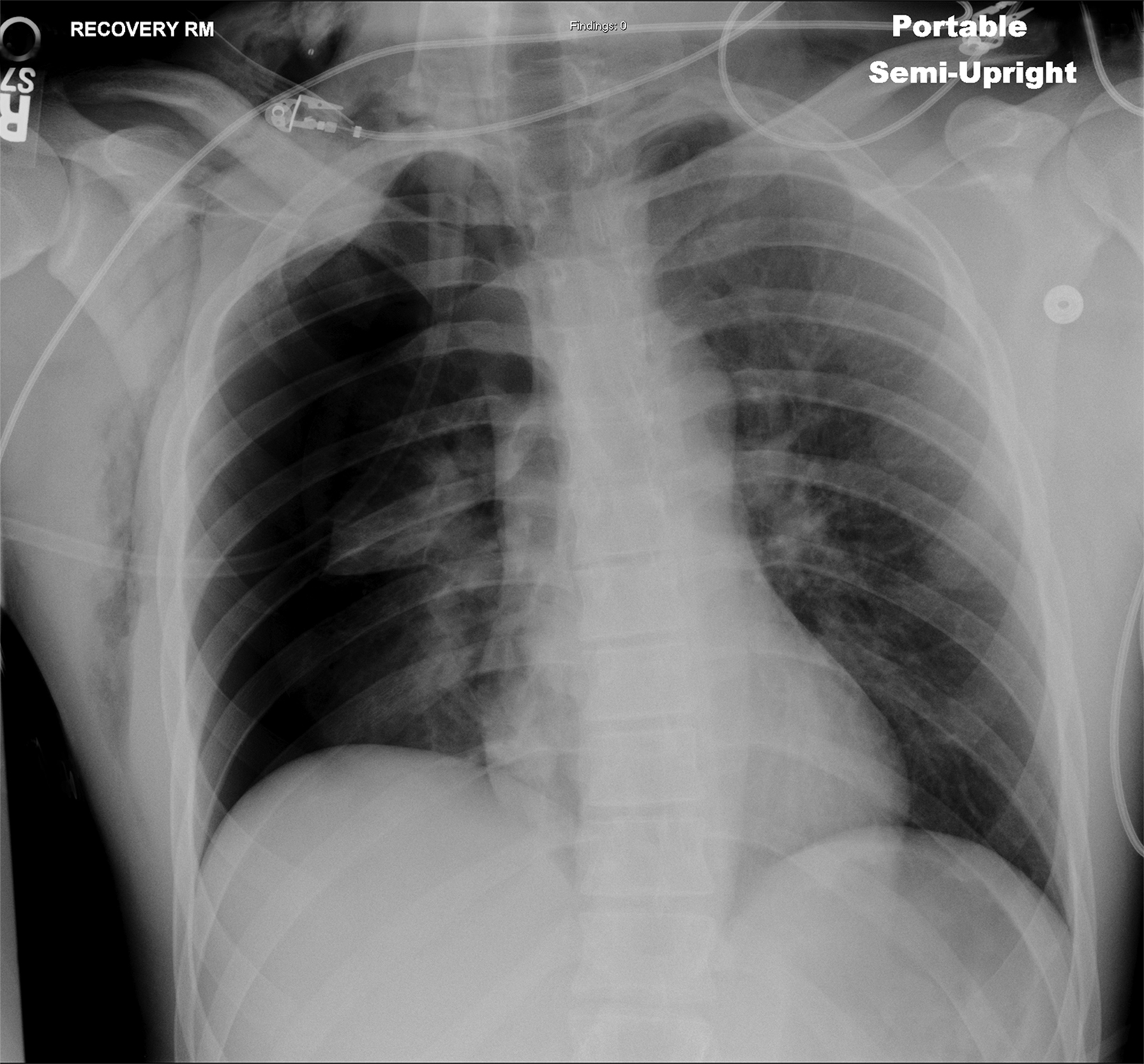
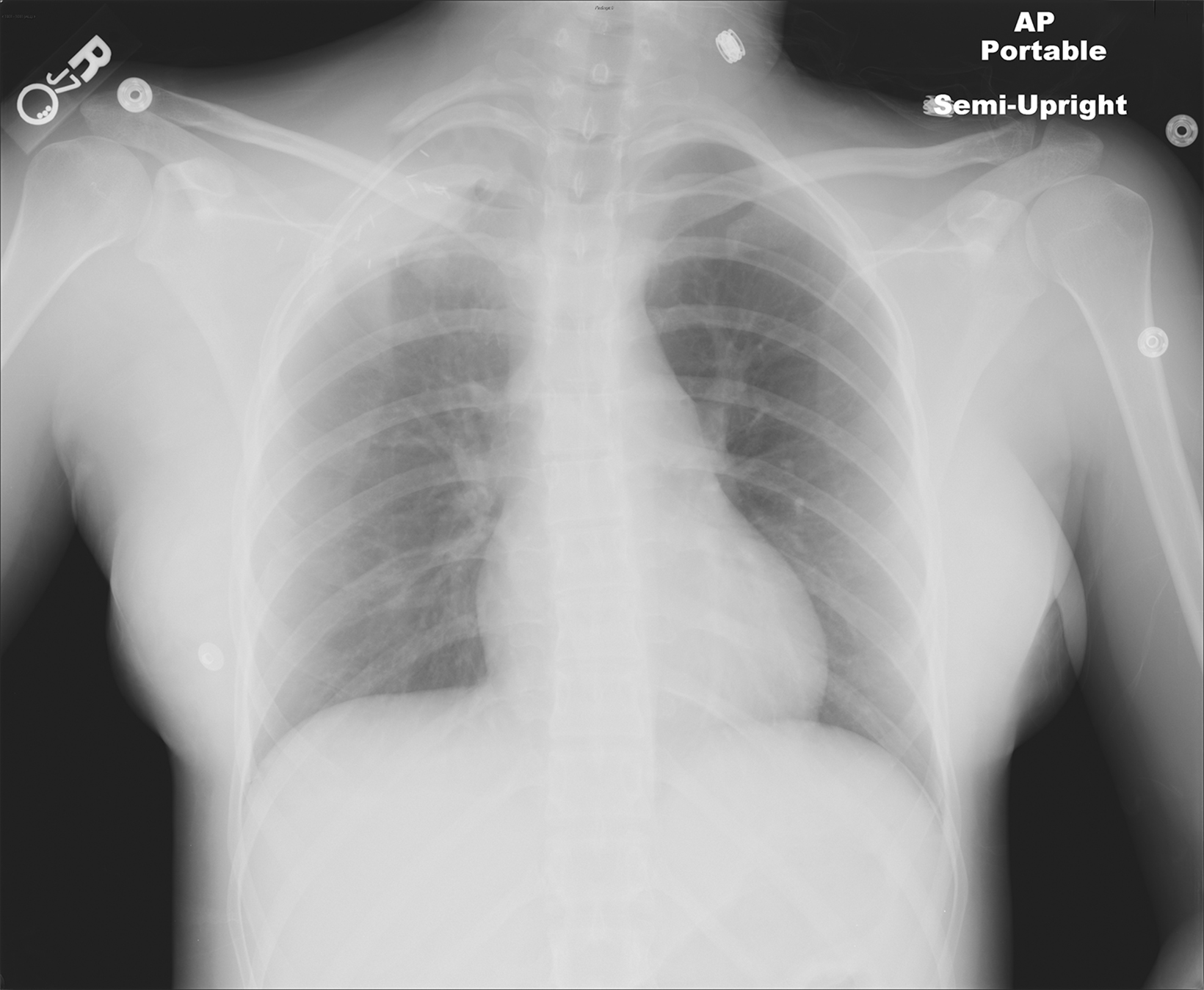
In the early postoperative period, radiographs will demonstrate an elevated hemidiaphragm ipsilateral to the surgical site (Figure 3). Follow-up radiography should be undertaken. A fluoroscopic sniff test can confirm the diagnosis of hemidiaphragmatic paralysis due to phrenic nerve injury and should be recommended in the setting of a persistently elevated hemidiaphragm following thoracic outlet decompression.
Pneumothorax
The most common complication of first-rib resection is a pneumothorax, with an incidence that has been reported as high as 33%.8 The parietal pleura lines the undersurface of the rib cage and may be easily violated during dissection and resection of the first rib, resulting in a pneumothorax.
Accumulation of air in the pleural space appears as an ipsilateral peripheral lucency on chest radiography (Figure 3). Mediastinal shift is indicative of a tension pneumothorax. If a pneumothorax is recognized in the operating room, a chest tube can be inserted into the pleural apex and the pneumothorax decompressed with suction.
A follow-up radiograph must be obtained to ensure re-expansion of the lung and proper chest-tube placement. In most instances, the pneumothorax is not recognized intraoperatively and a routine postoperative semi-upright chest radiograph will provide the first opportunity for detection. A small pneumothorax may be monitored with repeat chest radiography, pulse oximetry, and supplemental oxygen therapy. However, a large pneumothorax requires tube thoracostomy for evacuation.9
A tension pneumothorax requires immediate decompression with needle thoracostomy and emergent chest tube placement. The surgical team should be notified promptly when a pneumothorax is apparent on a postoperative chest radiography.
Chylothorax
The thoracic duct normally joins the venous system at the junction of the left internal jugular vein with the left subclavian vein. Therefore, the thoracic duct is susceptible to injury during thoracic outlet decompressions on the left side, resulting in leakage of lymphatic fluid into the pleural space. Radiographically, a chylothorax appears as an ipsilateral pleural effusion (Figure 3). Given anatomic variation of the location of the thoracic duct, bilateral chylothoraces are also possible.
Clinically, a chylothorax will present as a recurrent yellowish colored pleural effusion which will not resolve with chest tube placement. Loss can exceed 1500 mL per day. A slow lymphatic leak can result in significant soft tissue swelling and cause respiratory compromise. Additionally, a chylothorax can cause progressive electrolyte imbalance, as well as nutritional and immune deficiency, and is associated with a mortality rate greater than 50% if diagnosis and treatment is delayed.
Measuring triglycerides in the pleural fluid is supportive of the diagnosis. A chylothorax may resolve spontaneously with proper diet control or may require return to the operating room for thoracic duct ligation.7,10,11 Additional therapeutic options include interventional treatments such as thoracic duct embolization and therapeutic lymphangiography.12 The diagnosis of chylothorax should be considered in the setting of a persistent left pleural effusion following a left-sided TOD.
Hemothorax
Postoperative hemothorax generally occurs secondary to injury of the subclavian vessels. After first-rib resection, osseous fragments or sharp ends of bone have the potential to puncture the adjacent vasculature. The subclavian vein is more fragile than the subclavian artery.
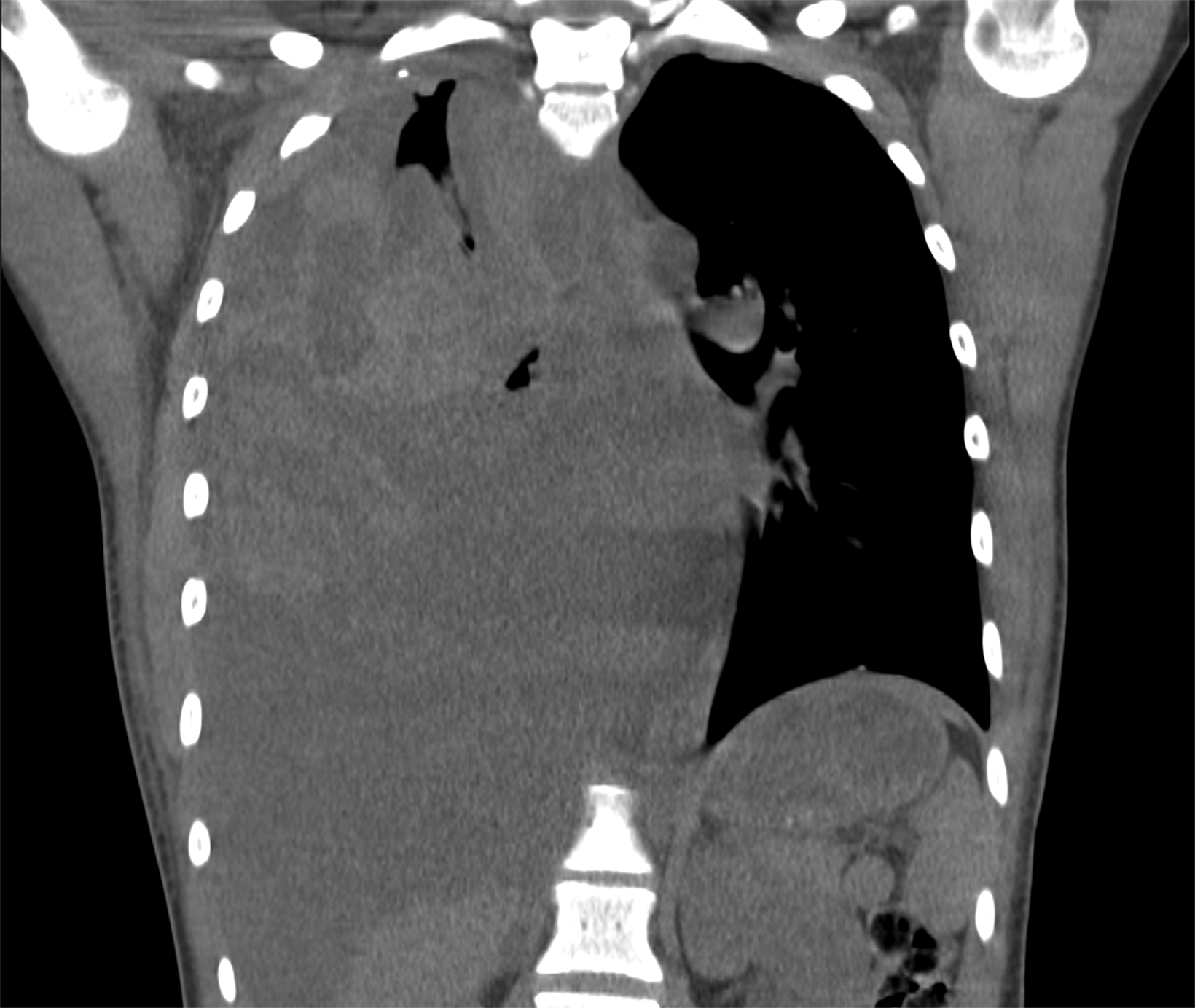
Accumulation of blood in the pleural space will appear as an ipsilateral pleural effusion on a postoperative plain radiograph (Figure 3).10 Chest computed tomography (CT) (Figure 3) can also help identify hemothorax as blood has higher attenuation values than the more simple fluid of a pleural effusion or the pus in an empyema.13
Although significant bleeding is rare with a reported incidence of 1.4%, a large hemothorax can cause hemodynamic instability and require emergent return to the operative room for definitive vascular repair and evacuation of the hemothorax.4,14,15 Thus, a new ipsilateral pleural effusion detected on plain radiograph in the immediate postoperative period should be promptly communicated with the surgical team and the diagnosis of hemothorax considered.
Nonsurgical management usually involves chest tube placement, and a follow-up CT may be valuable to ensure hemothorax resolution prior to chest tube removal.
Extrapleural Hematoma
An extrapleural hematoma results from injury to small vascular beds in the soft tissues within the operative field, causing accumulation of blood superficial to the parietal pleura. The resulting hematoma may be detectable on a postoperative plain radiograph as an apical opacity with convex margins projecting from the chest wall (Figure 3), caused by inward displacement of extrapleural fat.16 These injuries are not addressed with chest tubes as they are not in the pleural space.
Small hematomas may resolve spontaneously within 7-10 days and be of minor clinical significance.8 However, close monitoring with serial radiography and possible chest CT are recommended, since an enlarging apical hematoma often requires prompt surgical evacuation.7,9
Conclusions
Abnormal compression of the neurovascular structures within the thoracic outlet can result in one of the three subtypes of TOS: neurogenic, arterial, or venous.
In select patients, surgical decompression of the thoracic outlet with first- (and/or cervical) rib resection and scalenectomy is performed to alleviate symptoms and prevent sequelae of compression.
The specific surgical approach and technique are dictated by the subtype of disease. Nearly 75% of patients report an excellent functional outcome after TOD, and operative morbidity and mortality are low.6
Radiographically detectable complications of TOD include phrenic nerve injury, pneumothorax, chylothorax, hemothorax, and extrapleural hematoma. The first opportunity for detection of a surgical complication may arise on the routine postoperative chest radiograph. Thus, the radiologist plays an important role in the early postoperative evaluation of these patients.
Effective communication of pertinent findings may assist the surgeon in management of postoperative complications, especially in the critical setting of tension pneumothorax or in the case of broadening the differential diagnosis of pleural effusion to include hemothorax or chylothorax as clinically appropriate.
References
- >Chang DC, Lidor AO, Matsen SL et al. Reported in-hospital complications following rib resections for neurogenic thoracic outlet syndrome. Ann Casc Surg. 2007; 21:564-570.
- Thompson JF, Jannsen F. Thoracic outlet syndromes. Br J Surg. 1996; 83(4):435-436.
- Freischlag J, Orion K. Understanding thoracic outlet syndrome. Scientifica. 2014:248163.
- Urschel HC, Kourlis H Thoracic outlet syndrome: a 50-year experience at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2007; 20:125-135.
- Sanders RJ, Hammond, SL and Rao NM. Thoracic outlet syndrome: a review. Neurologist. 2008; 14(6):365-373.
- Desai SS, Toliyat M, Charlton-Ouw KM, et al. Outcomes of surgical paraclavicular thoracic outlet decompression. Ann Vasc Surg. 2014. 28(2):457-464.
- Atasoy E. A hand surgeon’s further experience with thoracic outlet compression syndrome. J Hand Surg Am. 2010; 35(9):1528-1538.
- Leffert RD. Complications of surgery for thoracic outlet syndrome. Hand Clin. 2004; 20:91-98.
- Balci AE, Balci TA, Cakir O, et al. Surgical treatment of thoracic outlet syndrome: effect and results of surgery. Ann Thorac Surg. 2003; 75:1091-1096.
- Hempel GK, Shutze WP, Anderson JF, et al. 770 Consecutive supraclavicular first rib resections for thoracic outlet syndrome. Ann Vasc Surg. 1996;10:456-463.
- Karamustafaoglu YA, Yoruk Y, Tarladacalisir T, et al. Transaxillary approach for thoracic outlet syndrome: results of surgery. Thorac Cardiov Surg. 2011; 59:349-352.
- Lyon SL, Mott N, Koukournaras J, et al. Role of interventional radiology in the management of chylothorax: a review of the current management of high output chylothorax. Cardiovasc Intervent Radiol. 2013; 36:599-607.
- Liu, F., Huang, Y. C., Yu-Bun, N., et al. Differentiate pleural effusion from hemothorax after blunt chest trauma; comparison of computed tomography at- tenuation values. Journal of Acute Medicine. 2016; 6(1):1-6.
- Dale WA. Thoracic outlet compression syndrome: critique in 1982. Arch Surg. 1982; 117(11):1437-1445.
- Gelabert HA, Jimenez JC, Davis GR, et al. (2011) Early postoperative hemorrhage after first rib resection for vascular thoracic outlet syndrome. Ann Vasc Surg. 25:624-629.
- Lewis B, Herr KD, Hamlin SA, et al. Imaging manifestations of chest trauma. Radiographics. 2021; 41(5):1321-1334.
