Coccidioidomycosis mimicking peritoneal carcinomatosis
By Devis P, Jindal R, Hur S, Eldersveld J


CASE SUMMARY
A 43-year-old previously healthy female presented to her primary care physician with a two-week history of 10-lb weight gain, increased abdominal girth, abdominal pain and fatigue. Her past medical history was largely unremarkable. Of note, she had lived in Arizona for three years. She was admitted to expedite her workup, and a bedside ultrasound revealed large-volume ascites. Aspirated ascitic fluid did not yield malignant cells, and cultures were negative for mycobacterial, acid-fast bacilli, or aerobic and anaerobic bacteria. A CT of the abdomen and pelvis was performed; an MRI of the chest, abdomen and pelvis was prompted due to the CT findings.
IMAGING FINDINGS
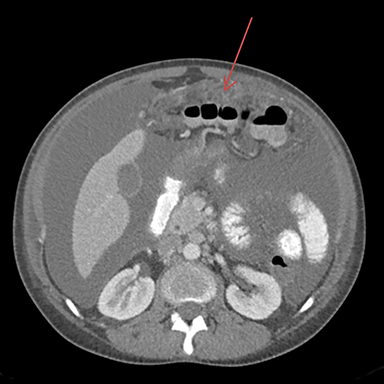
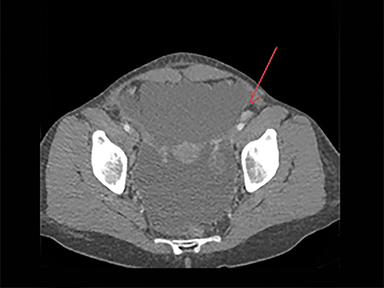
The CT of the abdomen and pelvis showed large-volume ascites with omental caking, as well as epicardial, and common iliac lymphadenopathy. No adnexal mass was identified. These findings resembled widespread metastatic disease without known primary (Figures 1-3).
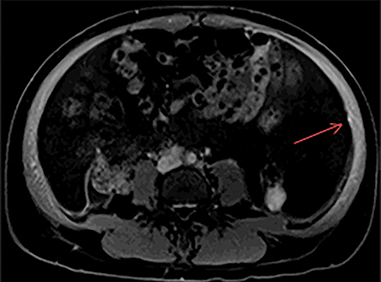
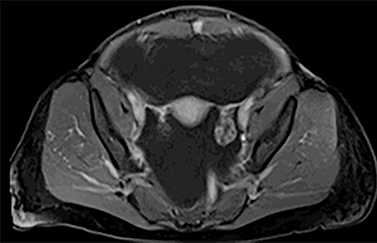
The MRI of chest, abdomen, and pelvis again demonstrated lymphadenopathy, including enlarged hilar lymph nodes, large volume abdominal ascites, omental caking and multiple enhancing nodules along the peritoneal lining (Figure 4). Additionally, it revealed an enhancing nodule within the rectus muscle (Figure 5). No adnexal mass was identified.
Endobronchial ultrasound guided biopsy of the hilar nodes was negative for malignant cells, no granulomatous disease was identified.
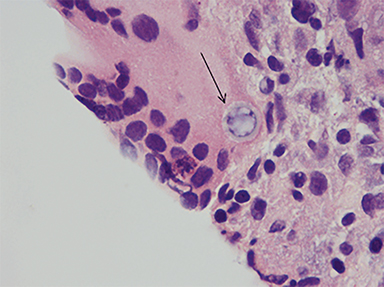
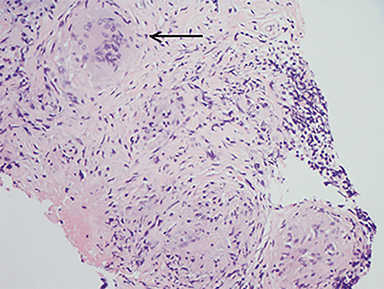
An inguinal lymph node and omental nodule biopsy were then performed by interventional radiology. Histopathologic analyses demonstrated necrotizing granulomatous reaction, rare spherules and multinucleated giant cells (Figures 6-8).
DIAGNOSIS
Disseminated coccidioides infection. Differential diagnosis includes widespread metastatic disease, lung primary was considered due to hilar lymphadenopathy; primary peritoneal carcinomatosis; tuberculosis; and atypical or fungal infection, such as coccidioidomycosis (in endemic areas).
DISCUSSION
Coccidioidomycosis, also known as the Valley Fever, is caused by a dimorphic fungus known as coccidioides – succinctly called “cocci” amongst clinicians and patients in the endemic areas. Within the United States, cocci is highly endemic in the southwestern areas (southern Arizona, the southern and central valleys of California, southwestern New Mexico, and west Texas). According to the United States Centers for Disease Control and Prevention, the incidence of coccidioidomycosis increased from 5.3 cases per 100,000 population in 1998 to 42.6 cases per 100, 000 population in 2011.1 In 2013, a total of 9,438 cases of valley fever were reported, 62 percent of which were from Arizona.2 In areas where valley fever is highly endemic, the valley fever remains an under-diagnosed condition.3
Infection is caused by inhalation of spores. Once in lung tissue, the mold undergoes significant morphological changes to form yeast, maturing into typical, thin-walled spherules, which rupture and release numerous endospores. Each endospore is capable of producing additional spherules.4 Repeated cycles of endospore release and spherule formation result in a granulocytic response. With progression of the inflammatory response, an epithelioid granuloma containing large histiocytes and giant cells develops. This is followed by central necrosis and a variable degree of fibrosis.5,6,7
Coccidioidomycosis can present with a wide range of clinical and radiological findings. Symptomatic cases typically present with symptoms of mild-to-severe acute pulmonary infection that resolves spontaneously. A small percentage of cases do develop serious manifestations of the disease, in which symptoms fail to resolve after two months of initial infection. In such cases, progressive pulmonary coccioidomycosis may present with nodular or cavitary lung lesions, cavitary lung disease with fibrosis, and miliary pulmonary dissemination with non-specific clinical and radiologic manifestations.6,7 The infection may also disseminate from the lung to other body parts, such as the central nervous system, skin, or bones and joints.8,9
CONCLUSION
New onset ascites, lymphadenopathy, omental caking, and peritoneal nodules typically point to metastatic carcinomatosis. However, in endemic areas, coccidioidomycosis also should be considered as a potential diagnosis. In our case, the lack of primary mass despite thorough investigation, the soft tissue nodules within the rectus muscle, and disseminated yet sparse lymphadenopathy did suggest an atypical or fungal infectious etiology, in addition to malignancy.
Coccidioidomycosis mimicking lung cancer in the form of pulmonary nodules and mass has been described extensively in the literature. CNS manifestations are also encountered in our medical center. This particular case of peritoneal presentation highlights coccidioidomycosis’s diverse clinical and radiographical presentations.
Our patient was started on a fluconazole regimen. Upon clinical follow up approximately 1 year later, she demonstrates no clinical signs of ascites and has returned to work. She will continue on the anti-fungal regimen for 2 more years.
REFERENCES
- CDC. Increase in reported Coccidioidomycosis – United States, 1998-2012. MMWR. 2013;62(12):217-221.
- CDC. Valley fever (Coccidioidomycosis) statistics: Fungal diseases. Centers for Disease Control and Prevention. 2015;http://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html.
- Valdivia L, Nix D, Wright M, Lindberg E, Fagan T, Lieberman D, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12(6):958-62.
- Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45:26.
- Desai NR, McGoey R, Troxclair D, Simeone F, Palomino J. Coccidioidomycosis in nonendemic area: Case series and review of literature. J La State Med Soc. 2010;162:97-103.
- Capone D, Marchiori E, Wanke B et al. Acute pulmonary Coccidioidomycosis: CT findings in 15 patients. Br J Radiol. 2008;81:721-724.
- Dues Filho A. Chapter 2: Coccidioidomycosis. J Bras Pneumol. 2009;35: 920-930.
- Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. Coccidioidomycosis: A descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine. 2004;83:149-175.
- Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217-1223.
Prepared by Dr. Devis while serving as Assistant Professor of Diagnostic and Vascular and Interventional Radiology in the Department of Medical Imaging at Banner University Medical Center, Tucson, AZ; Dr. Jindal while an MD candidate class of 2016 at the University of Arizona College of Medicine, Tucson, AZ; Dr. Hur while a Radiology Resident in the Department of Medical Imaging; and Dr. Eldersveld while a Pathology Resident in the Department of Pathology at Banner University Medical Center, Tucson, AZ.
