3D imaging in the angiography suite advances interventional patient care
Images



Supplement to Applied Radiology December 2012, sponsored by Philips
Dr. Siegel is System Chief, Interventional Radiology Services, North Shore LIJ Health System, and Associate Professor of Radiology, Hofstra North Shore LIJ School of Medicine, New Hyde Park, NY.
Interventional radiology has evolved rapidly over the last two to three decades, primarily due to refinements in catheters and catheter-based devices. These technological advances have allowed for the development of new techniques and applications of interventional therapy in territories previously not reachable. While image quality has improved and digital technology has been used to its full advantage over time in the evolution of angiography and interventional radiology suites, until recently little had really changed with respect to the way imaging has been utilized to guide these interventional procedures.
Cone-beam computed tomography (CT), developed several years ago, has revolutionized the way we guide procedures by allowing for soft-tissue imaging in the angiography suite that can be used with fluoroscopy. While rotating C-arms and 3-dimensional (3D) acquisition techniques were developed nearly 20 years ago, current technology adds the ability to image soft tissue with CT, along with improvements in fluoroscopic and angiographic imaging of contrast-filled vessels and other structures. Techniques for software reconstruction, manipulation, and analysis continue to be refined, and they now aid the interventional radiologist in guiding both vascular and nonvascular procedures in ways unimaginable as recently as 5 to 7 years ago.
At the forefront of the development of this technology is Philips Healthcare, whose flagship interventional suite is the Allura Xper FD 20 system. Besides providing the high-quality fluoroscopy and digital x-ray acquisition systems now customary in modern interventional suites, the ceilingsuspended C-arm of the Allura Xper FD 20 system can perform high-speed rotational scanning with or without simultaneous contrast injection, depending on the situation. This digital image dataset is then processed in seconds; depending on the technique utilized, the dataset provides interventionalists with a 3D vascular or soft-tissue image for diagnosis and 3D road mapping. Using the dedicated XperGuide software, this dataset also can be used to guide percutaneous interventions with the aid of interactive needle-path planning and guidance software. This sophisticated software overlays a preplanned needle path, which the operator designs at an integrated workstation. Previously acquired CT scans and images from other modalities, such as magnetic resonance imaging (MRI) and MR angiography, can also be imported and superimposed on a fluoroscopic image.
This article reviews the different abilities of the 3D tools available in newer interventional suites and provides an overview of their various clinical applications.
3D rotational angiography and road mapping
Rotational angiography takes advantage of the C-arm’s ability to rotate rapidly around the patient and acquire angiographic images at numerous oblique projections around its arch. Contrast injection volume and duration must be coordinated with the rotation speed and the desired images. Angiographers understand that the ability to see a vessel’s origination or the exact point and angle of branching is essential to planning procedures that require selective catheterization and precise endovascular therapy. The 3D reconstructed angiogram can also be used for 3D road mapping. The 3D image can be superimposed on the live fluoroscopic image and manipulated together with the live fluoroscopic image. Oblique angles can be obtained, the patient can be moved, and the image can be magnified during endovascular manipulations and interventions. Previously, numerous stationary oblique “runs” were required, using trial and error; once the appropriate projection was determined, it was then employed for treatment planning and guidance.
With practice, interventionalists gain an understanding of when the added time, contrast, and radiation of these rotational acquisitions will ultimately lead to lower cumulative procedural time, contrast use, and exposure.
Cone-beam CT
Cone-beam CT employs image acquisition similar to that of rotational angiography. Computer software then performs a sophisticated 3D reconstruction, resulting in images that can be viewed as a multiplanar reconstruction. These images can be manipulated, rotated and zoomed; adjustments in window and level also can be made. Imaging soft tissue simultaneously with opacified vessels can be essential to appreciating the relationship of these structures and understanding the blood supply and drainage of various organs. In interventional oncological procedures, when caustic chemotherapeutic preparations or radioactive particles are to be introduced into the liver vessels, confining the materials within the liver is essential, as non-target embolization can be catastrophic, especially when it involves the GI tract. If a vessel is opacified during such a procedure and its vascular territory is uncertain, XperCT can be performed during contrast injection, and the vascular distribution identified on that soft-tissue imaging. These techniques can be utilized outside the liver, as well. We often utilize cone-beam CT before embolization to evaluate the potential distribution of the embolic. Following embolization procedures, XperCT can assess the precise territory embolized, making it clear whether further embolization is necessary.1
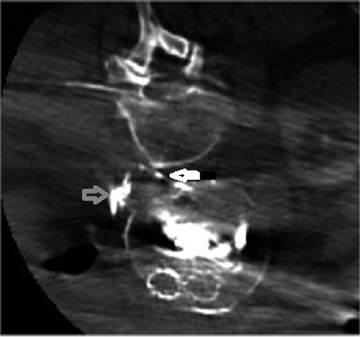
Understanding the relationship of vessels to surrounding structures can be essential to diagnosing different vascular conditions. Paget-Schroetter syndrome, or thoracic outlet syndrome, is a condition where the subclavian vessels are crushed between the first rib and the clavicle and confined by the scalene muscles between those bony structures. This generally occurs when the affected arm is abducted and extended. Figure 1 is a cone-beam CT image that demonstrates Paget-Schroetter syndrome. On this image, compression of the subclavian artery by the surrounding structures is beautifully depicted.
XperCT can also be employed during interventional procedures to locate and evaluate devices. We have used cone-beam CT imaging to guide filter placement in patients with severe contrast allergies or renal failure, to guide fenestration of aortic dissections by locating the appropriate point for flap puncture, and to evaluate the course of catheters or guide wires when it is unclear if the true lumen of an occluded vessel was traversed or if a collateral vessel that would be dangerous to dilate was catheterized. The applications for this technology continue to expand.
Interventional tools for needle guidance
The 3D image dataset obtained by the cone-beam CT acquisition of the Philips FD20 Allura Xper unit can be used with the dedicated needle- guidance software to plan a needle path and then to aid the operator in precisely placing the needle during a variety of interventional procedures. The 3D CT image is first used to design the course of a needle or multiple needles that do not traverse any significant vascular or other dangerous structures. The unit will then assume the necessary compound oblique positions based on calculated coordinates. Initially, the C-arm will assume a “down the barrel” projection, or target view, and superimpose a circle on the fluoroscopic field where the needle should be placed. After fluoroscopically guided placement of the needle, so that only a point is seen, the C-arm is then turned to an orthogonal view to monitor progress of the advancing needle. When the unit is turned to this orthogonal view, or to any position, the 3D soft-tissue image and needle path remain superimposed on the fluoroscopic image. Biopsies and other procedures requiring needle access can be performed more accurately and reliably, translating into fewer needle passes and lower complication rates, especially when related to bleeding and post-procedure discomfort. Figure 2 is a target view for a biopsy of a 19-year-old man with a benign cartilaginous lesion of the iliac bone. Radiation dose
Radiation exposure to the patient is certainly a factor in deciding how and when cone-beam CT and/or 3D angiography should be utilized in interventional practice. While rotational C-arm imaging techniques certainly deliver a greater radiation dose to the patient than does conventional fluoroscopy, in many situations, this technology can actually dramatically decrease the total fluoroscopy time and number of individual digital acquisitions—therefore decreasing overall radiation exposure.
When a needle can be advanced under real-time fluoroscopic guidance after a single cone-beam CT acquisition, the need for interval CT scanning during manipulations and needle passes is eliminated. Even with the addition of an extra CT scan to confirm needle position, cumulative radiation dose to a patient during a complex biopsy or other procedure requiring CT guidance is usually decreased. For these situations, the Philips Allura Xper FD20 system allows for a lower-dose cone-beam CT acquisition. This will produce an image of somewhat lower quality, but it can be used to determine needle position accurately. The overall decrease in radiation to patients during biopsy procedures has been validated in several published studies.2
Complex interventional procedures
We now use XperGuide in many clinical situations where accurate CT-needle guidance placement is needed in conjunction with additional vascular or nonvascular catheter and guide wire-based procedures. The combination of soft-tissue CT imaging, needle-guidance software, and 3D angiographic imaging can often simplify what would be relatively complex or cumbersome procedures; at times, it eliminates the need to move a patient from one suite to another where these different modalities are available. There are several reports of translumbar endoleak embolization utilizing cone-beam CT guidance for sac puncture.3 Figure 3 demonstrates an example of XperCT following endoleak embolization using coils, a vascular plug, and tissue acrylic.
We regularly perform nephrostomy placement, biliary drainage, and complex fluid collection drainage with Xper guide. Needle placement can be guided with the accuracy of CT imaging in an environment where subsequent catheter manipulations and exchanges can be performed with high-quality fluoroscopic guidance. Needle guidance has significantly expanded our interventional armamentarium. For example, we have performed puncture and intubation of the pancreatic duct for stenting of a persistent leak. Utilizing overlay of an MR image for targeting the cisterna chili, we were able to access the thoracic duct and then embolize a postoperative leak. Figure 4 demonstrates an intraprocedural XperCT with MR overlay, obtained in a patient with a venous malformation where recurrent symptoms were related to a deeper, previously untreated loculation. This deeper portion of the malformation was targeted and successfully treated with sclerotherapy, resulting in complete symptom relief.
Conclusion
The availability of 3D angiographic and CT imaging with needle-guidance software in the traditional interventional environment brings us one step closer to the full-service, image-guided procedure suite, where interventional radiologists can perform all procedures with the required technology at their disposal. Future developments in this technology should continue to enhance our precision and expand the role of interventional medicine.
Acknowledgement: The author would like to thank his colleague, Igor Lobko, MD, for his collaboration in much of the work discussed in this article.
References
- Tognolini Alessia, Louie John D., Hwang Gloria L., et al. Utility of C-arm CT in patients with hepatocellular carcinoma undergoing transhepatic arterial chemoembolization. J Vasc Interv Radiol. 2010;21: 339-347.
- Braak Sicco J, Strijen van Marco JL, Es van Hendrik W, et al. Effective dose during needle interventions: Cone-beam CT guidance compared with conventional CT guidance. J Vasc Interv Radiol. 2011;22:455-461.
- Bindsbergen van Lars, Braak Sicco J, Strijen van Marco JL, de Vries Jean-Paul PM. Type II endoleak embolization after endovascular abdominal aortic aneurysm repair with use of real-time three-dimensional fluoroscopic needle guidance. J Vasc Interv Radiol. 2010;21:1443-1447.