A diagnostic approach to splenic lesions
Images





















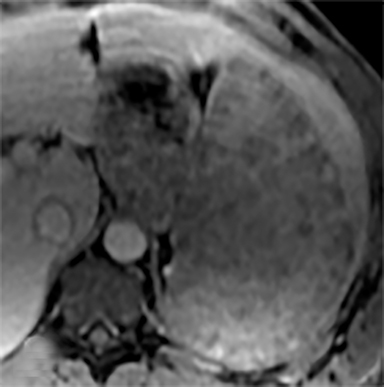
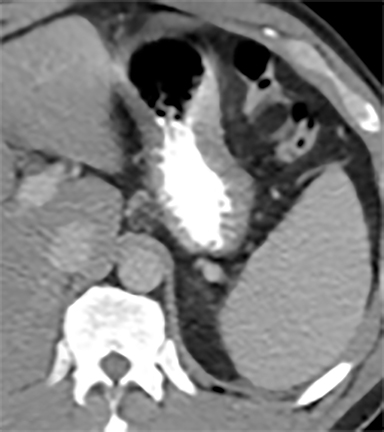
Most splenic lesions are detected incidentally, posing a challenge for both interpreting and referring physicians in determining the need for and type of further evaluation. Paluska et al1 found incidental splenic lesions in 1% of patients in an emergency room population undergoing computed tomography (CT) scans for abdominal pain or trauma.1 Most of these were considered clinically benign. However, in patients with a known malignancy or symptoms attributable to possible splenic pathology, the incidentally discovered splenic lesion may be more significant. Clinical factors must be taken into account when evaluating a splenic lesion, most importantly pain attributable to the spleen, signs and symptoms of infection, immune status, history of known malignancy, associated findings on imaging of the chest, abdomen or pelvis and a history of abdominal trauma, either recent or remote. Certain laboratory values, such as white blood cell count, can also provide valuable information to narrow the differential diagnosis. In this article, the authors propose an algorithm based on clinical factors for narrowing the differential diagnosis of an incidental splenic mass.
Spleen histology and imaging features
The spleen is an encapsulated organ composed of vascular sinuses, which comprise the red pulp, and interspersed cords of lymphatic tissue, making up the white pulp.2 CT imaging demonstrates heterogeneous splenic enhancement on arterial phase imaging, due to variation in blood flow through the sinuses and cords. In the portal venous phase, splenic enhancement becomes homogeneous. On magnetic resonance imaging (MRI), the normal spleen demonstrates lower signal intensity than the liver on T1-weighted images (T1WI) and higher signal intensity than liver on T2-weighted images (T2WI). The enhancement pattern is similar to its appearance on CT, with heterogeneous enhancement in the arterial phase and homogeneous enhancement in the portal venous phase.
Diagnostic considerations
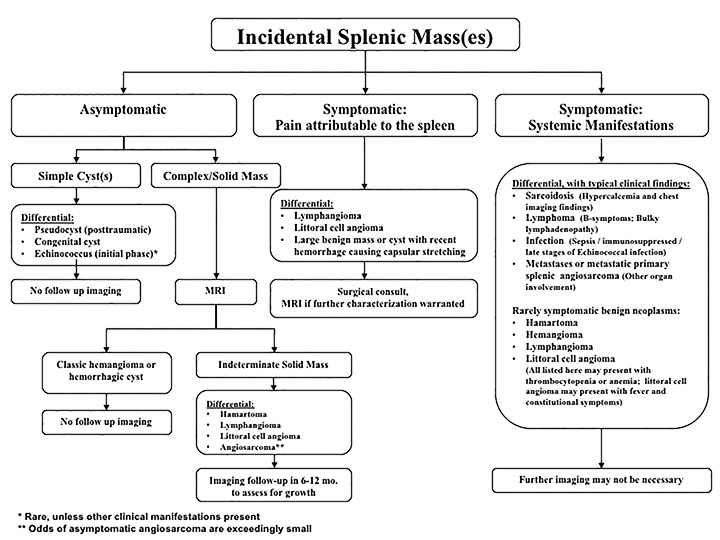
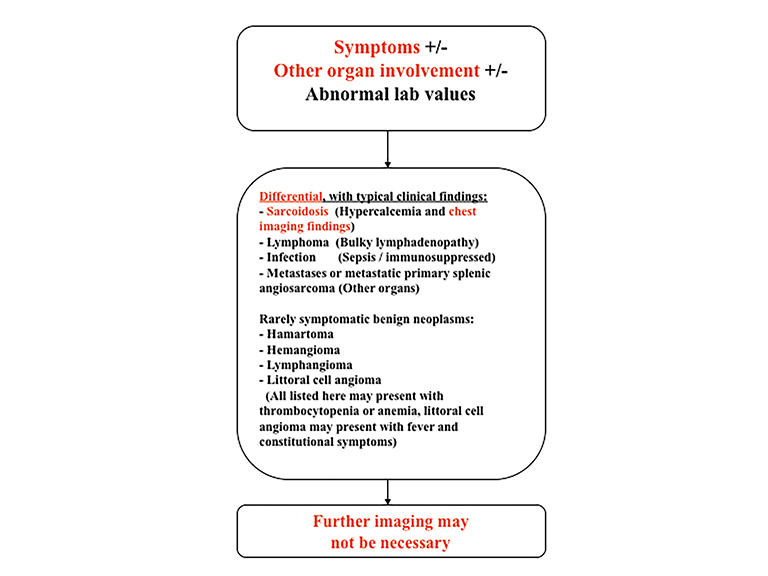
The differential diagnosis of focal splenic lesions can be divided into several categories. These include cystic lesions, primary vascular neoplasms, infectious or inflammatory processes, lymphoproliferative disorders, and metastases. Examples of primary neoplasms include hemangioma, hamartoma, lymphangioma, littoral cell angioma, hemangioendothelioma, and angiosarcoma. Infectious etiologies encompass bacterial, fungal and mycobacterial abscesses. Sarcoidosis is a multisystem inflammatory process that can present with splenic lesions in addition to other organ involvement as the lungs and mediastinum. Combining imaging findings with the clinical assessment facilitates the formulation of a more relevant differential diagnosis. Our proposed algorithm, based on clinical presentation, is shown in Figure 1. Patients are triaged initially into one of three categories: asymptomatic, symptomatic with pain attributable to the spleen, or symptomatic with systemic involvement. Subsequent figures demonstrate the utility of the proposed algorithm in an asymptomatic and symptomatic patient with additional organ involvement (Figures 23-26).
Asymptomatic
As previously noted, most splenic lesions are found incidentally. The majority of these lesions are benign and clinically insignificant. Heller et al described a management algorithm based on imaging characteristics, prior imaging studies and clinical history.3 If a splenic lesion with benign imaging characteristics (either a cyst or homogeneous, low-attenuation with no enhancement and smooth margins) is detected in an asymptomatic patient, no follow-up imaging is necessary. If the imaging features are not diagnostic, stability based on prior imaging also negates further work-up. However, if the imaging features are not diagnostic and there are no prior imaging studies available for comparison, further evaluation or follow-up imaging is warranted based on either a clinical history of malignancy or the observed imaging findings. Our algorithm divides simple cysts from complex cysts/solid masses. The differential diagnosis for simple cysts includes pseudocysts, congenital or hydatid cysts. Cystic lesions need no further evaluation or follow-up imaging.
Simple cysts
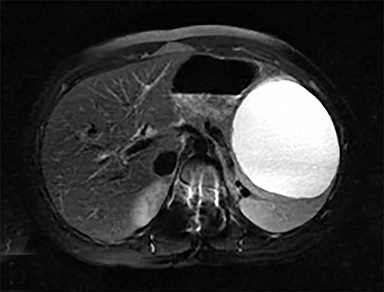
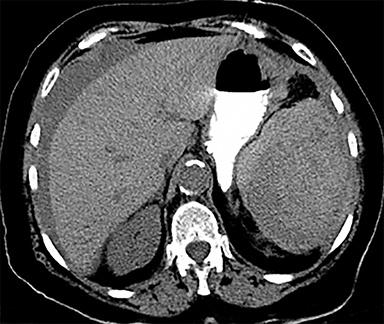
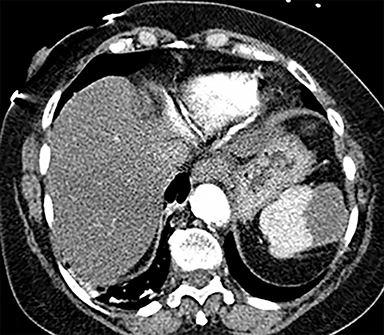
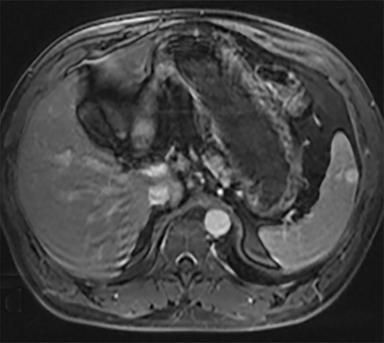
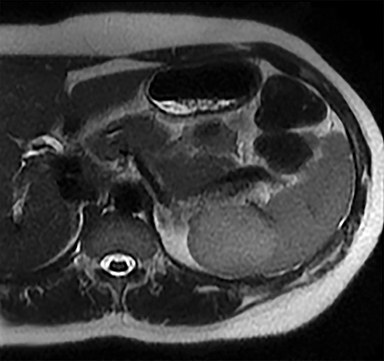
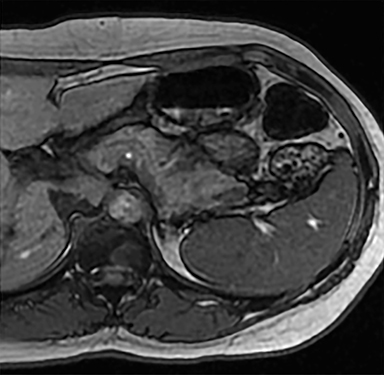
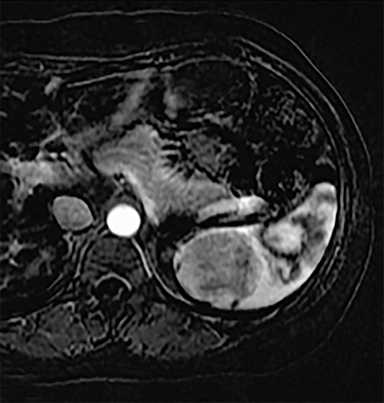
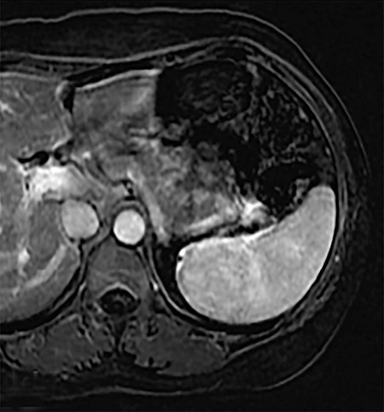
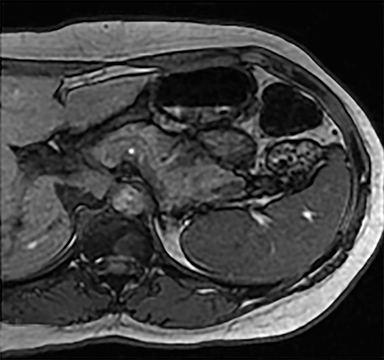
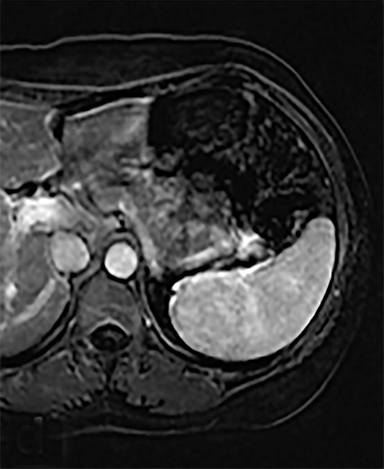
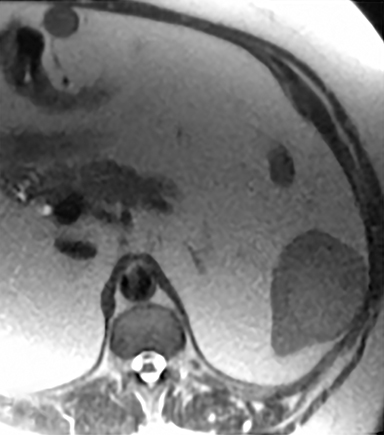
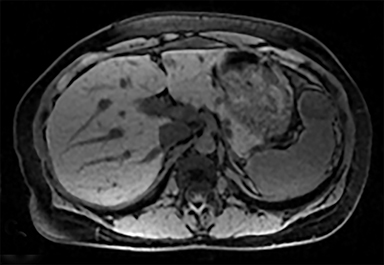
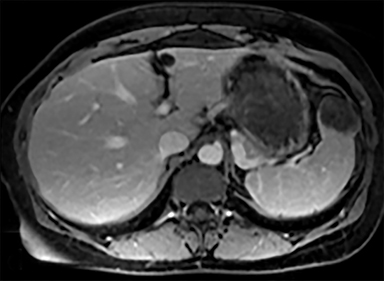
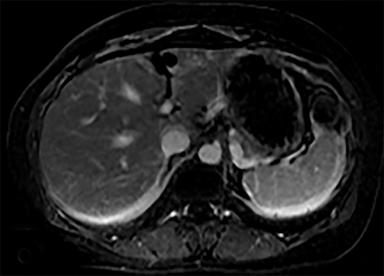
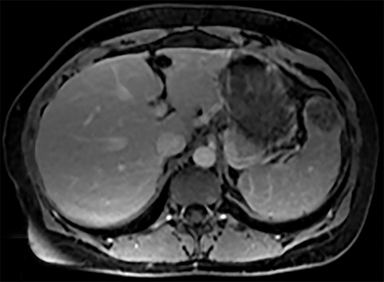
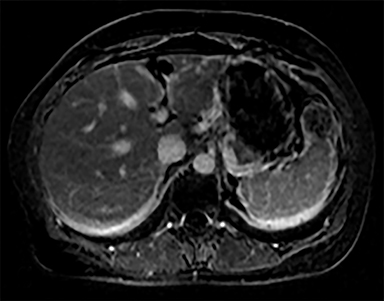
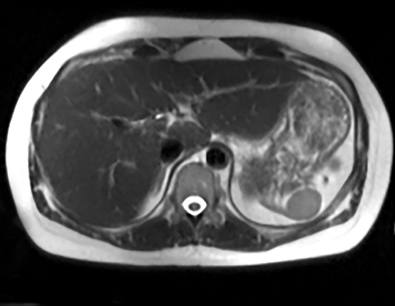
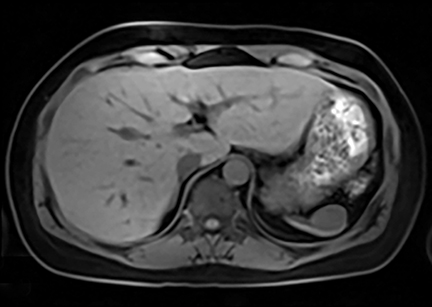
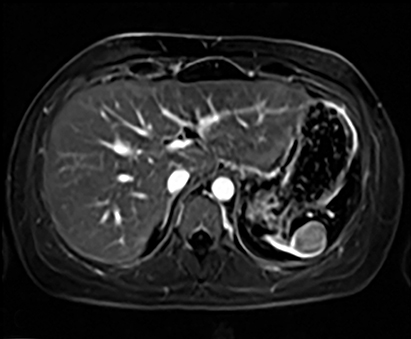
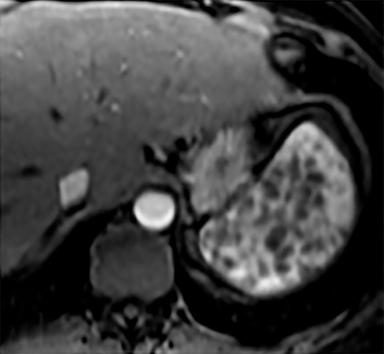
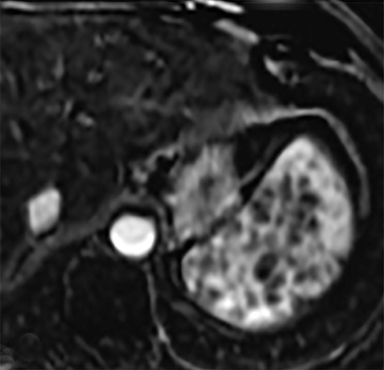
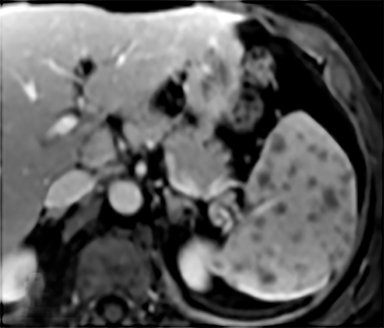
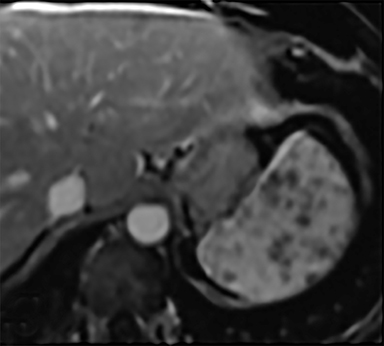
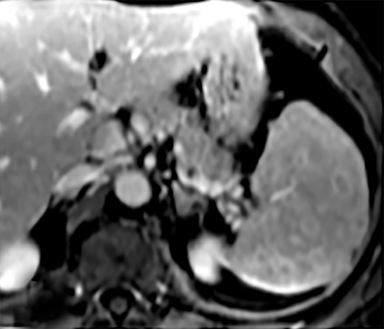
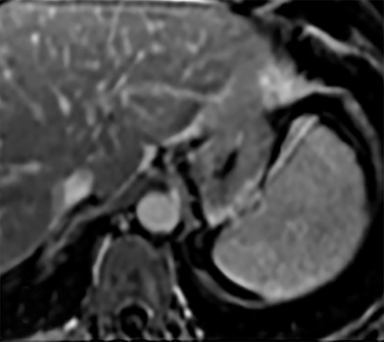
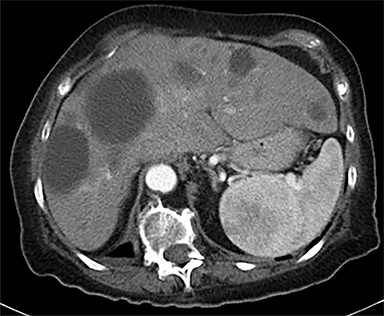
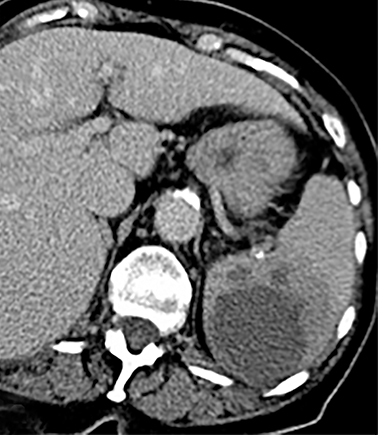
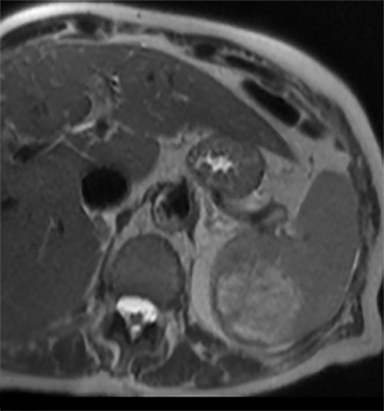
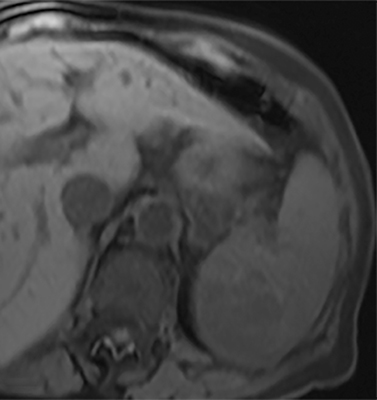
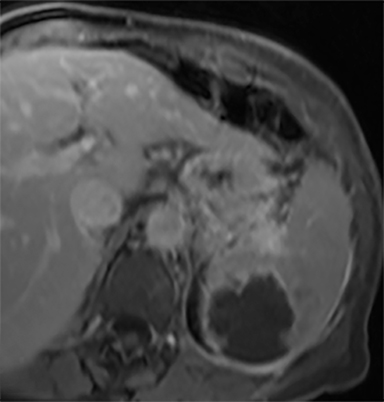
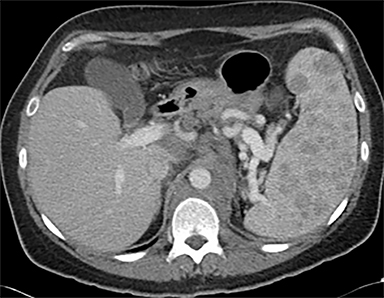
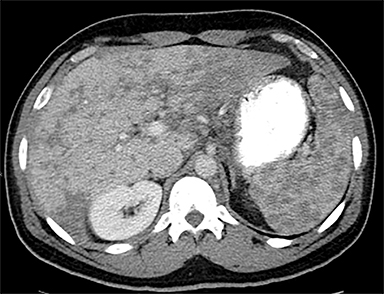
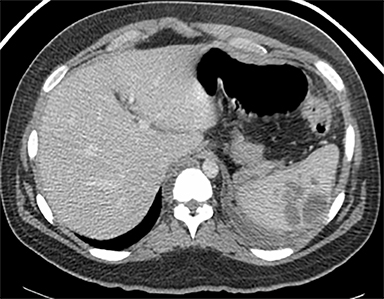
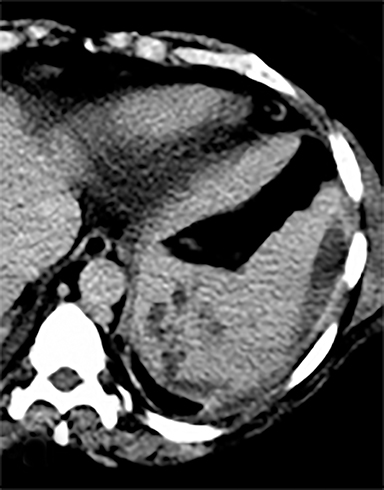
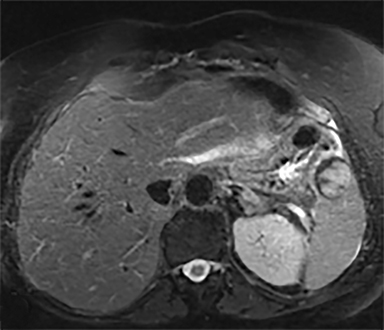
Cysts of the spleen are the most common benign focal splenic masses. They are typically asymptomatic, but may enlarge and become painful. There are two types of cysts: true cysts and pseudocysts. Pseudocysts are much more common, representing 80% of all splenic cysts. Also known as post-traumatic cysts, these cysts do not contain an epithelial lining and can develop as a sequela of prior trauma, infarction or infection. Mural calcification occurs in the wall in 30-40% of cases.4 The other 20% of cysts possess an epithelial lining and are considered true cysts.5 These may be congenital or caused by parasitic infections, such as Echinooccocus. Echinococcal cysts can be indistinguishable from posttraumatic and congenital cysts, but are exceedingly rare in North America. When present, these patients are usually symptomatic and are discussed below. On CT, splenic cysts are sharply demarcated, typically unilocular and hypoattenuating (Figure 2A), and do not enhance after contrast administration (Figures 2A and 3). They may demonstrate higher attenuation when containing protein, hemorrhage, or superimposed infection. The walls of cysts are thin unless infected or when associated with recent hemorrhage. Cysts on MRI are typically hypointense to splenic parenchyma on T1WI and hyperintense on T2WI, although signal intensity can also vary in the presence of protein or hemorrhage (Figure 2B). As on CT, cysts do not enhance (Figure 2C). If determined to be simple cysts on CT or MRI, no follow-up imaging is necessary.
Complex cystic or solid masses
Another category of focal splenic lesions in the asymptomatic patient is the complex cystic or solid masses. In this group, contrast enhanced MRI is needed for further evaluation. If the lesion has signal and enhancement characteristics of a hemangioma or hemorrhagic cyst, no follow-up imaging is necessary. However, if indeterminate on MRI, follow-up imaging in 6-12 months is recommended to monitor stability. The differential diagnosis for indeterminate solid masses includes hamartoma, lymphangioma, littoral cell angioma, and angiosarcoma. Most patients with angiosarcomas present with systemic manifestations from additional organ involvement and are discussed below.
Hemorrhagic cysts
A potential, albeit rare, complication of splenic cyst is hemorrhage. On CT, hemorrhagic cysts may be hyperdense and can exhibit fluid-fluid levels. On MR imaging, hemorrhagic cysts usually demonstrate high signal on T1WI from internal contents and may show fluid-fluid levels. There is no enhancement and these lesions do not require follow-up.
Hemangioma
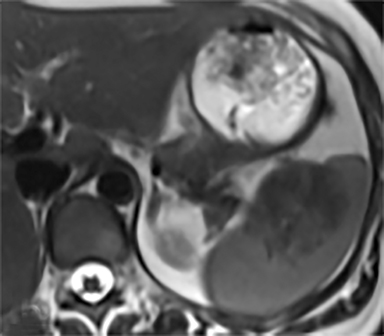
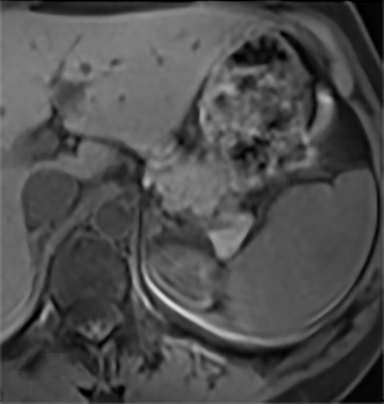
The most common benign primary neoplasm of the spleen is a hemangioma. These tumors form by proliferation of vascular channels, ranging from small capillaries to large cavernous types.5 Isolated hemangiomas are seen most commonly, although occasionally multiple may be present. Most hemangiomas are asymptomatic and incidentally discovered. These lesions are hypoattenuating on nocontrast CT. Homogenous or peripheral enhancement occurs after contrast administration, (Figure 4A) which progresses over time on later post-contrast phases. The discontinuous, peripheral, centripetal enhancement pattern seen in other hemangiomas, such as in the liver, is less common in splenic hemangiomas. Hemangiomas are hypo- to isointense to splenic parenchyma on T1WI and hyperintense on T2WI (Figure 4). The enhancement patterns on MRI are similar to that of CT (Figures 4, 5) Multiple splenic hemangioma can be associated with Kasabach-Merritt syndrome or Klippel-Trenaunay-Weber syndrome.6 Splenic hemangiomas are benign and do not need follow-up to monitor stability.
Hamartoma
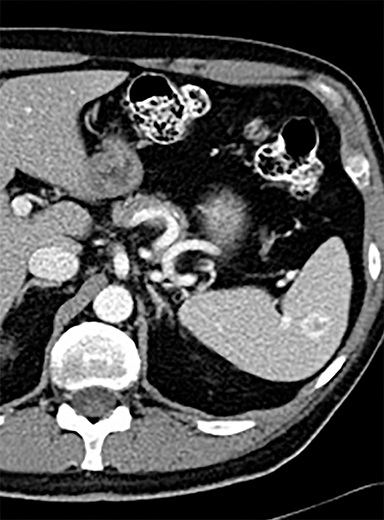
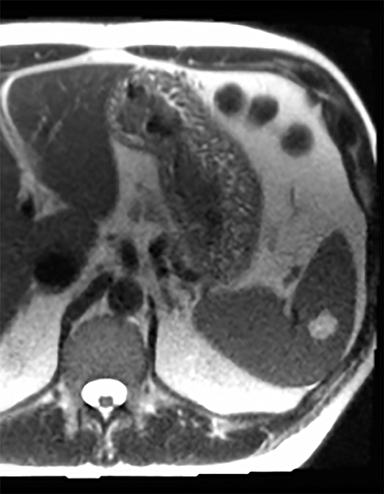
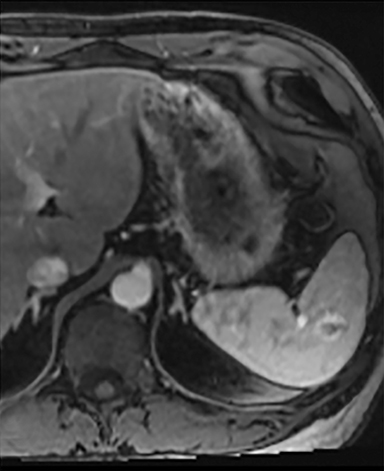
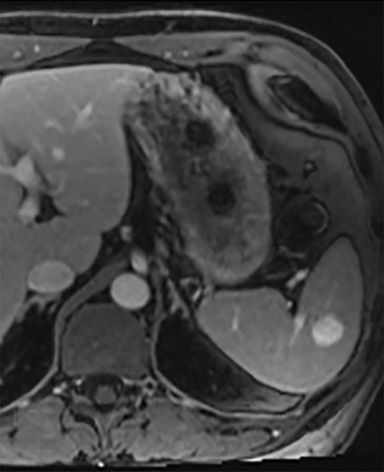
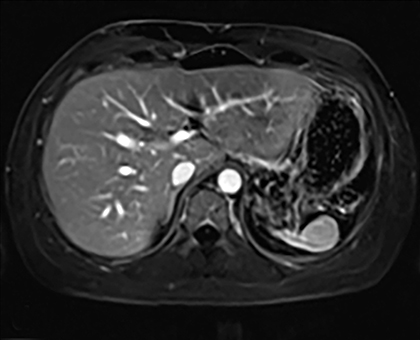
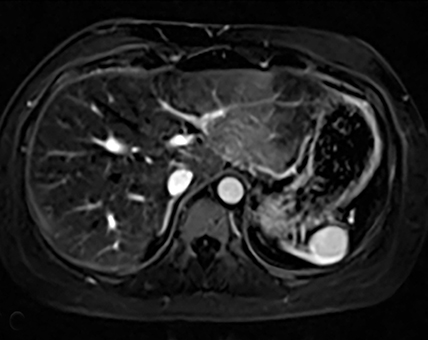
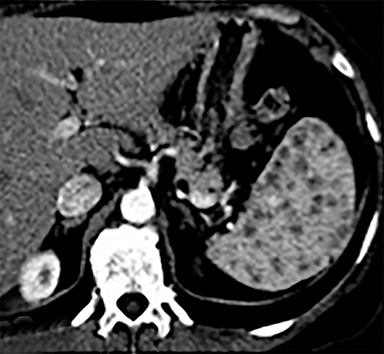
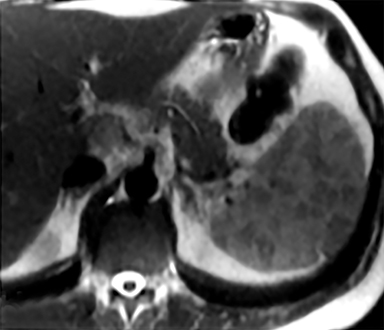
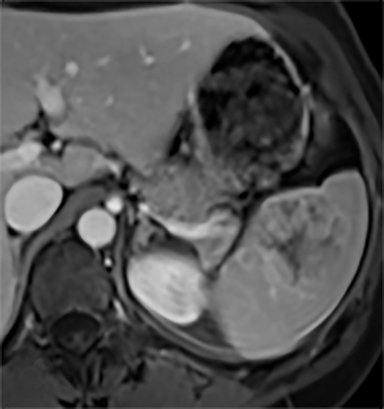
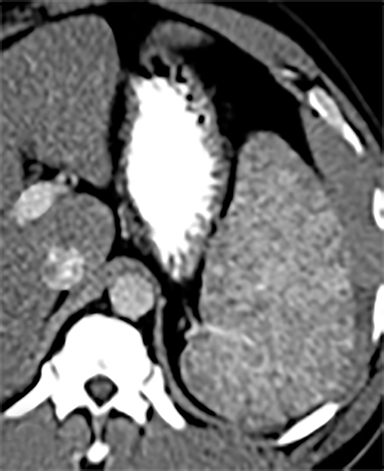
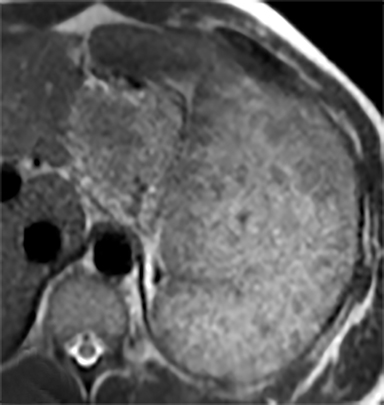
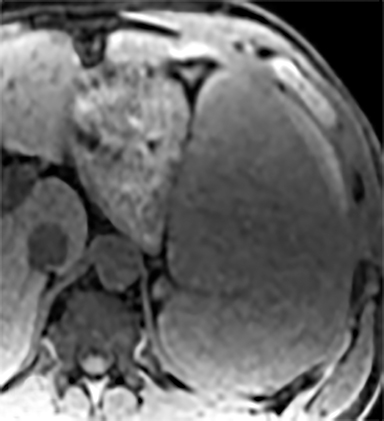
Splenic hamartomas are mixtures of disorganized vascular channels and fibrotic red or white pulp elements.5 Often, these are congenital, though sometimes acquired lesions that occur as a proliferative or a neoplastic process. Most hamartomas are asymptomatic, but if large enough, patients may present with a palpable mass or pain due to mass effect and stretching of the splenic capsule. In extremely rare cases, splenic hamartomas may present with symptoms secondary to rupture. They can be associated with tuberous sclerosis and Wiskott-Aldrich-like syndrome5. On CT, hamartomas are commonly isoattenuating, bulging masses, which cause focal contour deformity of the spleen. They can enhance diffusely or heterogeneously. On T1WI, hamartomas are isointense to splenic parenchyma and are mildly hyperintense and heterogeneous on T2WI (Figure 6A, 6B). Enhancement may be diffuse or heterogeneous, as on CT (Figure 6C, 6D). On delayed post contrast imaging, hamartoma may exhibit hyperenhancement or may be isointense with the spleen parenchyma (Figure 7). No imaging follow-up is necessary for splenic hamartomas if confident diagnosis can be made by imaging.
Symptomatic lesions: Pain attributable to the spleen
In this group of focal splenic lesions, patients present with left upper quadrant pain due to mass effect and stretching of the splenic capsule. As shown in Figure 1, there is overlap of several lesions in this category in the asymptomatic solid mass group. Often these lesions are asymptomatic when small and may become painful as they enlarge. Surgical consultation should be considered when abdominal pain is potentially attributable to a splenic mass. If clinically warranted, further characterization with MRI may be helpful to narrow the differential diagnosis or to assess for aggressive imaging features that may change management.
Lymphangioma
A lymphangioma is a congenital abnormality of lymphatic tissue. Different subtypes of lymphangiomas exist, including capillary, cavernous and cystic types: the latter being the most common. In the spleen, these may be solitary, scattered or diffuse. Patients may be asymptomatic or present with symptoms related to mass effect. Complications include hemorrhage, coagulopathy, hypersplenism, and portal hypertension. In children, these tumors may be part of a systemic lymphangiomatosis syndrome, which involves the mediastinum, lungs, liver, kidney, GI and GU tracts.5 Surgical resection is necessary only when lymphangiomas are symptomatic. On CT, these appear as hypoattenuating masses that do not enhance with contrast (Figure 8A). CT may detect peripheral wall calcifications, which are most often present in the cystic type. On T2WI they are hyperintense with hypointense septations (Figure 8B). MRI characteristics of lymphangioma include low T1 signal, unless hemorrhagic (Figure 8C). On post contrast imaging, there is enhancement of the thin septations (Figures 8D-G).
Littoral cell angioma
Littoral cell angioma is a rare vascular splenic neoplasm arising from littoral cells, which line the splenic red pulp sinuses and believed to play a role in the immune response.5 Many of these tumors contain hemorrhagic products. Patients may be asymptomatic, although more often, patients present with abdominal pain, splenomegaly, anemia, thrombocytopenia and constitutional symptoms. Littoral cell angioma is typically benign, but malignant behavior has been reported rarely, including metastatic disease.7 Often these tumors are resected due to their nonspecific imaging characteristics or because of their symptomatic presentation. Most published studies describe multiple masses as a more common presentation, but in our experience, these tend to present as solitary masses. Typically, littoral cell angiomas are hypoattenuating to the splenic parenchyma on non-contrast CT. They remain hypoattenuating on arterial or early portal venous phase, but become isointense to spleen parenchyma on delayed imaging.5,7-9 On MRI, they are hypointense on T1WI and have variable signal intensity on T2WI depending on the presence of hemorrhagic products (Figure 9A and B). They are T1 hypointense on early post contrast images and iso- to hyperintense on delayed imaging (Figure 9C-E). These lesions can have an overlapping imaging appearance with hamartoma.
Symptomatic: systemic manifestations
Certain clinical and laboratory findings can help narrow the differential diagnosis of splenic lesions. Patients with sarcoidosis may have hypercalcemia or they may have preexisting pulmonary/mediastinal disease. Patients with lymphoma may present with B-symptoms (fever, night sweats and weight loss) or may have lymphadenopathy on physical examination. Infection has a wide array of clinical presentations, not limited to fever and leukocytosis. These patients may be septic, and often they are immunocompromised. The presence of lesions in other organs in addition to the spleen should raise suspicion for metastases or metastatic primary splenic angiosarcoma. Rarely, patients with benign neoplasms may present with abnormal laboratory values, such as thrombocytopenia or anemia. The differential diagnosis for this includes hamartoma, hemangioma, lymphangioma and littoral cell angioma. In addition, littoral cell angioma may present with fever and constitutional symptoms. In patients with splenic lesions and systemic manifestations, further imaging is usually not necessary and treatment is targeted to the underlying disease or conservative management is used for benign tumors.
Sarcoidosis
Sarcoidosis is characterized by non-caseating granuloma on histology. This disease primarily affects the lungs, with symptoms of dyspnea, wheezing and chest discomfort. Other organ involvement is much less common, with solely extrapulmonary findings found in only 10% of patients.5 Isolated splenic disease is quite rare. Splenic sarcoidosis may be associated with liver involvement and/or mediastinal and upper abdominal lymphadenopathy. Most patients with splenic involvement are asymptomatic, although sometimes patients may have symptoms related to hypercalcemia. Those that are asymptomatic usually do not require any therapeutic intervention. On CT, there may be diffuse hypoattenuating nodules without obvious enhancement (Figure 10A).2,10,11 MRI may show multiple, tiny hypovascular and hypointense nodules on all sequences, which may demonstrate minimal peripheral enhancement on delayed sequences (Figures 10B-H).2,12 A solitary splenic mass is a very unusual manifestation of splenic sarcoid (Figure 11).
Lymphoma
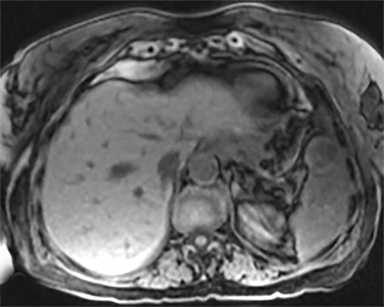
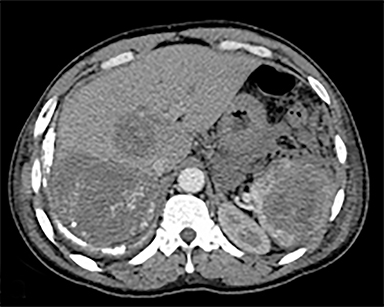
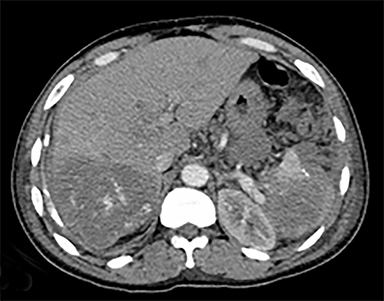
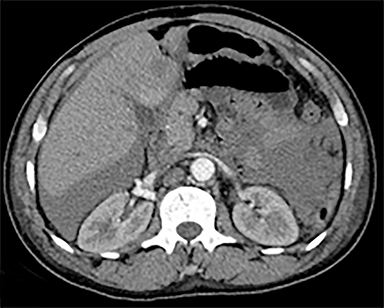
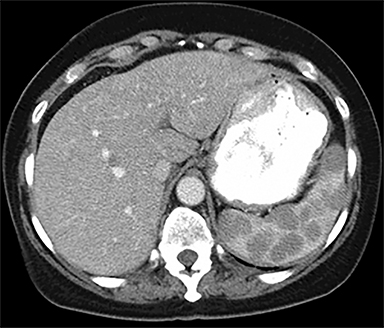
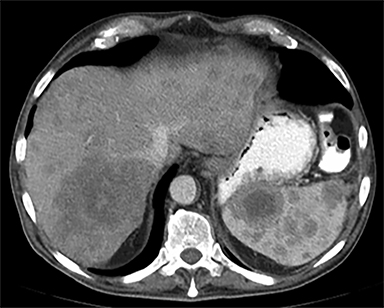
The most common malignant neoplasm of the spleen is lymphoma. Disseminated lymphomatous disease with spleen involvement is more common than primary splenic lymphoma. There are often multiple or diffuse nodules throughout the spleen and only rarely is a solitary splenic mass discovered. Retroperitoneal lymphadenopathy is a common associated finding, but this may not be present in primary splenic or extranodal lymphoma. On CT, the spleen appears diffusely enlarged, with or without areas of low attenuation (Figures 12-14).2 MRI imaging characteristics are similar to normal splenic parenchyma unless it is cystic, necrotic or hemorrhagic. Lymphoma is typically isointense on T1WI and iso- to hypointense on T2WI (Figure 12B and 12C). Early after contrast administration, nodules are hypointense and then become isointense to splenic parenchyma on delayed postcontrast imaging (Figure 12C and 12D).12
Infection
Infection is rarely isolated to the spleen, but most commonly seen with concurrent hepatic abscesses. Patients may present with fever and malaise, positive blood cultures and with lymphadenopathy on physical exam. Fungal micro abscesses are the most common form of splenic infection in an immunocompromised patient, including Candidiasis, Aspergillus, Cryptococcus, and Histoplasmosis (Figure 15).13 Splenic tuberculosis is characterized by irregular miliary disease or coalescent micronodules, which become focal masses as they increase in size (Figure 16).14 Hypoattenuation of the nodules on CT represents necrosis. There may be wedge-shaped regions of low attenuation on CT, representing infarcts related to septic emboli. When abscesses are organized and encapsulated, there may be peripheral enhancement on postcontrast images and septations may also enhance. The presence of internal gas is diagnostic but uncommon. MRI is rarely performed, given the diagnostic value of CT combined with clinical history (Figure 17). There may be value if the clinical picture is unclear and if other imaging such as CT is indeterminate. Infectious lesions are typically hypo to isointense on T1WI and hyperintense on T2WI (Figure 17).2 As on CT, there may be enhancement of a capsule or septations, if present. Splenic involvement in patients with Hydatid disease is rare (less than 2%). Patients usually present with abdominal pain, fever, and splenomegaly. The Echinococcal cyst consists of a dominant mother cyst containing smaller, peripherally located daughter cysts. Mural ring-like calcification of the walls of both the mother cyst and daughter cysts is common. These are usually treated surgically because of the risk of rupture.
Angiosarcoma
Angiosarcoma is histologically characterized by disorganized vascular channels.5 An extremely rare tumor, it constitutes less than 2% of soft tissue sarcomas. Angiosarcomas are aggressive, presenting late, with diffuse metastasis to the liver, lung, bones, and lymphatic system. They have been associated with prior chemotherapy for lymphoma or breast cancer.15,16 There is also a known association with prior use of Thorotrast, a radioactive contrast medium used for cerebral angiography from 1930-1960, which has a biological half-life of several hundred years and is retained in the reticuloendothelial system.17 Clinically, patients present with abdominal pain, fever, fatigue, weight loss, anemia, thrombocytopenia, and coagulopathy, with a risk of spontaneous rupture of the tumor in 30%.18 On MR, they are heterogeneous on both T1WI and T2WI due to the presence of hemorrhage and necrosis (Figure 18), with variable degrees of enhancement2. Masses are heterogeneously enhancing on CT with hyperattenuating areas of hemorrhage (Figure 19).2
Splenic metastases
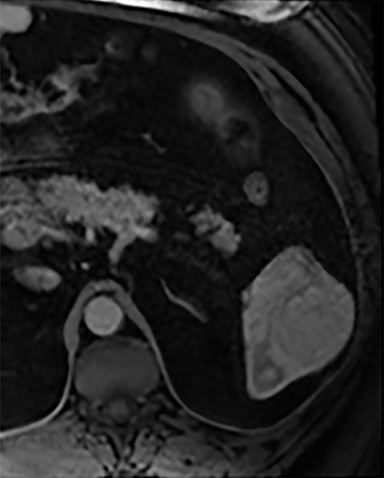
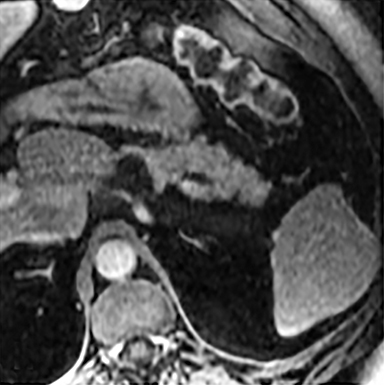
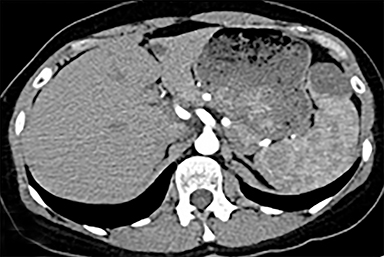
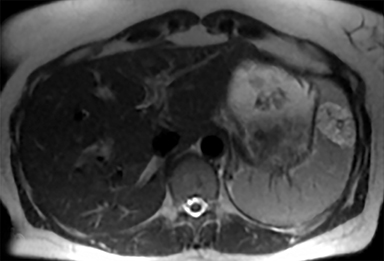
The spleen is a rare site for metastatic disease, with only a 3% occurrence in a large autopsy series.19 Some theories for its low incidence include the sharp angle of the splenic artery, absence of afferent lymphatics and high concentration of lymphatic tissue. When metastases to the spleen do occur, they are usually late in course of the disease, following involvement of multiple other organ systems. The most frequent primary neoplasms associated with splenic metastases are lung carcinoma, melanoma, and breast carcinoma given the higher occurrence of these malignancies overall. The highest frequency of splenic metastasis occurs with germ cell tumors, melanoma, and small cell lung carcinoma.20 On CT, metastatic lesions are typically hypoattenuating, often with a “target” appearance, and can demonstrate homogeneous or heterogeneous enhancement (Figures 20, 21). Isolated splenic metastases are much less common, and have been reported in patients with melanoma, colorectal, and ovarian carcinoma (Figure 22). MRI demonstrates hypo- to isointensity of the lesions on T1WI and mild hyperintensity on T2WI, unless there is a hemorrhagic component, in which case the metastatic foci may be T1 hyperintense and T2 hypo- to isointense. On postcontrast imaging, metastases demonstrate heterogeneous or rim-enhancement.12 Melanoma metastases can be hyperintense on T1WI, either secondary to hemorrhage or the intrinsic paramagnetic properties of melanin, and tend to be hypervascular on postcontrast images.
Role of splenic biopsy
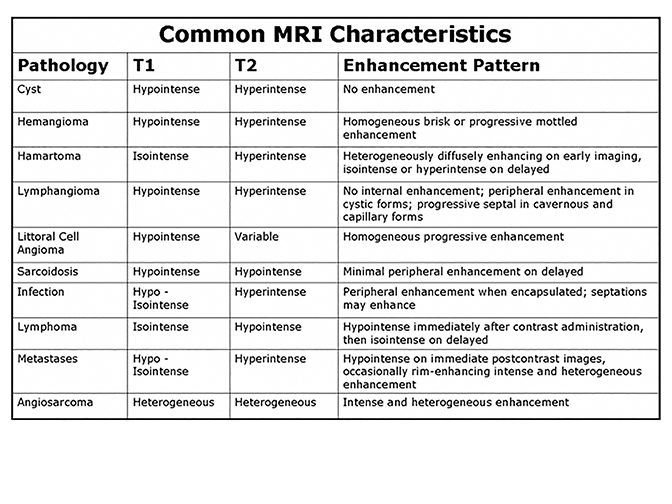
Percutaneous biopsy of the spleen is technically feasible with a complication rate comparable to biopsy of other intra-abdominal organs.21 Biopsy should be considered when there is an unknown primary diagnosis, if there is a concern for abscess and/or the imaging findings are atypical or nonspecific for a given diagnosis. Figure 27 summarizes MR findings typically seen for a variety of splenic pathology. As reflected in this review, MRI characteristics can be highly valuable in differentiating complex splenic masses, although significant overlaps exist. In such circumstances, a biopsy should be performed cautiously especially in patients with abnormal coagulation parameters. In the case of multi-organ involvement, a reasonable alternative is to sample a lesion in a less vascular organ.
Utility of FDG-PET/CT
PET/CT with 18-fluorodeoxyglucose (FDG) has a role for evaluating focal splenic lesions, especially in patients with a history of malignancy.22 In a patient with known malignancy, FDG PET/CT can be useful in differentiating malignant from benign lesions. If the mass is hypermetabolic, it should be considered malignant. Conversely, if the mass is non-FDG-avid, it is likely benign and may be followed with CT or MRI to assess stability. However, in a patient without a known malignancy, FDG-PET has virtually no utility in differentiating benign from malignant lesions. In these scenarios, FDG-PET could be helpful if the splenic mass increases in size. If the enlarging mass is hypermetabolic, tissue sampling is recommended. If not, careful inspection of the companion CT should be undertaken to look for non-FDG-avid lesions elsewhere to suggest a disseminated process, in which case biopsy of any lesion is recommended. If no additional CT findings are identified, continued follow-up is considered appropriate.
Extremely rare diagnoses
Inflammatory pseudotumor
There are a few extremely rare diagnoses in the spleen that present as focal lesions. One of them is the inflammatory pseudotumor, which is characterized histologically by inflammatory fibroblastic changes with granulomatous components.5 The etiology for inflammatory pseudotumor is uncertain.
Peliosis
Peliosis is another exceedingly rare diagnosis. Multiple blood-filled spaces without an endothelial lining are present.5 Peliosis is associated with anabolic steroid use. Hemangiopericytomas are tumors that represent a proliferation of pericytes in vascular channels. On imaging, they may demonstrate lobular contour with satellite lesions. Lastly, hemangioendotheliomas have variable histology and may contain necrosis or hemorrhage. Imaging characteristics for this rare tumor reflect these characteristics.
Conclusion
Focal splenic masses are rare, with no standard management protocol delineated in the literature. The majority of incidental lesions are benign, and correlation with clinical history is invaluable for diagnosis and management. An algorithm proposed in this article creates a systematic approach to incidentally encountered splenic masses. We believe that using this stepwise approach when evaluating splenic masses not only helps narrow the differential diagnosis, but also helps guide further management by the appropriate use of additional imaging, while avoiding unnecessary testing.
References
- Paluska TR, Sise MJ, Sack DI, et al. Incidental CT findings in trauma patients: incidence and implications for care of the injured. J Trauma. 2007;62(1):157-161.
- Rabushka LS, Kawashima A, Fishman EK. Imaging of the spleen: CT with supplemental MR examination. Radiographics. 1994;14(2):307-332.
- Heller MT, Harisinghani M, Neitlich JD, et al. Managing incidental findings on abdominal and pelvic CT and MRI, Part 3: white paper of the ACR Incidental Findings Committee II on splenic and nodal findings. J Am Coll Radiol. 2013;10(11):833-839.
- Webb WR, Brant WE, Major NM. Fundamentals of body CT. Philadelphia: Saunders; 2014.
- Abbott RM, Levy AD, Aguilera NS, et al. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24(4):1137-1163.
- Cha SH, Romeo MA, Neutze JA. Visceral manifestations of Klippel-Trénaunay syndrome. Radiographics. 2005;25(6):1694-1697.
- Falk S, Stutte HJ, Frizzera G. Littoral cell angioma: a novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15(11):1023-1033.
- Bhatt S, Huang J, Dogra V. Littoral cell angioma of the spleen. AJR Am J Roentgenol. 2007;188(5):1365-1366.
- Levy AD, Abbott RM, Abbondanzo SL. Littoral cell angioma of the spleen: CT features with clinicopathologic comparison. Radiology. 2004;230(2):485-490.
- Prabhakar HB, Rabinowitz CB, Gibbons FK, et al. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am J Roentgenol. 2008;190 (3_supplement):S1-S6.
- Warshauer DM, Lee JK. Imaging manifestations of abdominal sarcoidosis. AJR Am J Roentgenol. 2004;182(1):15-28.
- Luna A, Ribes R, Caro P, et al. MRI of focal splenic lesions without and with dynamic gadolinium enhancement. AJR Am J Roentgenol.. 2006;186(6):1533-1547.
- Ahmed S, Horton KM, Fishman EK. Splenic incidentalomas. Radiol Clin North Am. 2011;49(2):323-347.
- Bean MJ, Horton KM, Fishman EK. Concurrent focal hepatic and splenic lesions: a pictorial guide to differential diagnosis. J Comput Assist Tomogr. 2004;28(5):605-612.
- Falk S, Krishnan J, Meis J. Primary angiosarcoma of the spleen A clinicopathologic study of 40 cases. Am J Surg Pathol. 1993;17(10):959-970.
- Smith VC, Eisenberg BL, McDonald EC. Primary splenic angiosarcoma. Case report and literature review. Cancer. 1985;55(7):1625-1627.
- dos Santos Silva I, Malveiro F, Jones ME, et al. Mortality after radiological investigation with radioactive Thorotrast: a follow-up study of up to fifty years in Portugal. Radiat Res. 2003;159(4):521-534.
- Autry JR, Weitzner S. Hemangiosarcoma of spleen with spontaneous rupture. Cancer. 1975;35(2):534-539.
- Schön CA, Görg C, Ramaswamy A, et al. Splenic metastases in a large unselected autopsy series. Pathol Res Pract. 2006;202(5):351-356.
- Lam K, Tang V. Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch Pathol Lab Med. 2000;124(4):526-530.
- Lucey BC, Boland GW, Maher MM, et al. Percutaneous nonvascular splenic intervention: a 10-year review. AJR Am J Roentgenol. 2002;179(6):1591-1596.
- Metser U, Miller E, Kessler A, et al. Solid splenic masses: evaluation with 18F-FDG PET/CT. J Nucl Med. 2005;46(1):52-59.
Citation
D T, S S, M M, S M, J A, A K, D R. A diagnostic approach to splenic lesions. Appl Radiol. 2017; (2):7-22.
February 8, 2017