Abernethy malformation
Images
CASE SUMMARY
A 4-year-old girl presented with a 1-week history of fever, altered sensorium, generalized abdominal pain, and distention. She had a prior history of multiple hospitalizations since birth for repeated episodes of jaundice. Bloodwork showed raised total bilirubin levels (17.4mg/dl) with direct bilirubin 10.2 mg/dl & indirect bilirubin 7.2 mg/dl with markedly elevated WBC count of 15,900 /mm3. The blood ammonia levels were extremely high at 129 mcg/dl and serum galactose levels were raised.
IMAGING FINDINGS
A chest frontal view X-ray showed presence of inferior rib notching. Echocardiography demonstrated a juxtaductal coarctation of the aorta surgically corrected by an end-to-end anastomosis. Abdominal sonography revealed a vascular channel formed by the confluence of the splenic and superior mesenteric veins. This vessel coursed away from the gastro-hepatic ligament and opened into the infrahepatic inferior vena cava (IVC). The left and right branches of the portal vein and intrahepatic portal venules were absent. Color Doppler ultrasonography showed hepatofugal flow in this vascular channel.
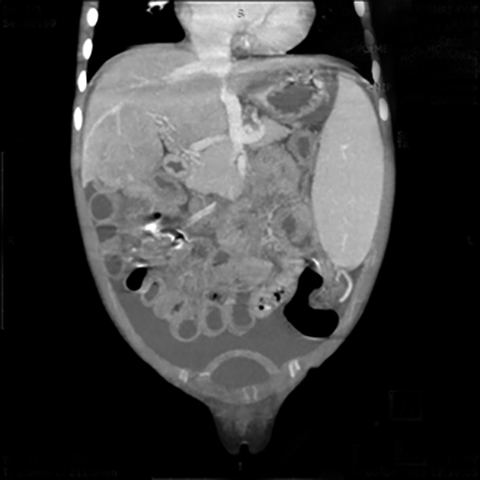
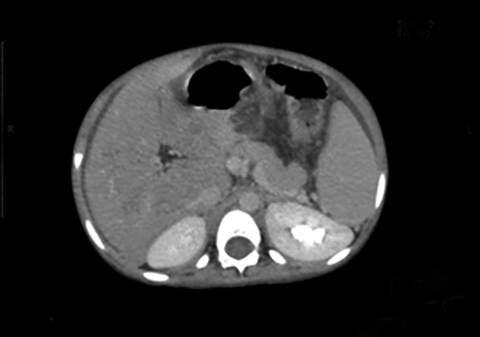
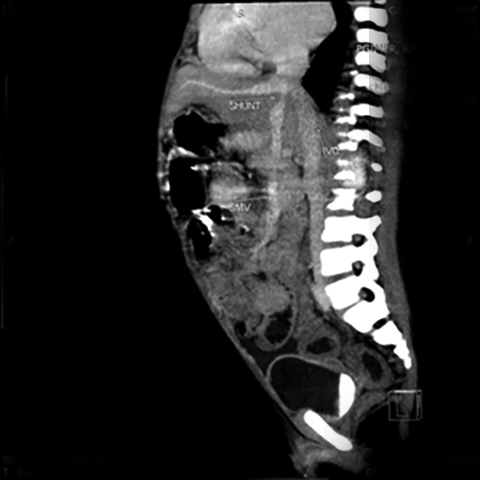
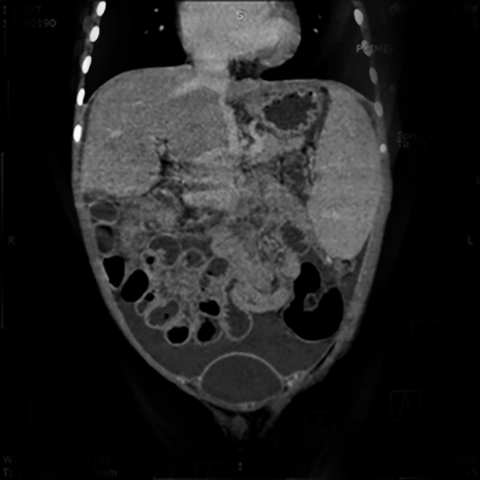
An abdominal contrast-enhanced CT scan was performed to evaluate this vascular channel. Axial images demonstrated formation of the portal vein by confluence of the splenic and superior mesenteric veins at the neck of pancreas, but instead of coursing into the liver through the porta hepatis, this vein formed an end-to-side anastomosis with the extra hepatic IVC (Figure 1). The portal vein was absent at the porta and the intrahepatic portal vein branches and venules were not seen (Figure 2). Multiplanar reconstructions allowed accurate depiction of this abnormal extra- hepatic mesenteric-caval shunt (Figure 3). CECT also showed moderate ascites with massive splenomegaly and smooth peritoneal enhancement (Figure 4).
DIAGNOSIS
Abernethy malformation (Type Ib CEPS)
DISCUSSION
Abernethy malformation is a congenital extra hepatic portosystemic shunt (CEPS) that develops between the porto-mesenteric vasculature and the systemic veins. 1 John Abernethy gave the first account of absent portal vein and congenital mesenteric-caval shunt. The basic abnormality is a vascular aberration in which the splanchnic venous return drains directly into the systemic veins (IVC or the left renal, left iliac or left hepatic vein), diverting the mesenteric circulation away from the liver. The portal vein is either hypoplastic or by-passes the liver.
The portal vein normally forms between weeks 5 and 10 of embryogenesis by selective involution of the peri-intestinal vitelline venous loop. The IVC, on the other hand, develops as an amalgamation of several embryologic venous channels with the common hepatic vein, which gives rise to the hepatic segment of the IVC. Excessive involution between fetal weeks 4 and 10 can result in complete absence of the portal vein, and this leads to CEPS with abnormal mesenteric-caval connection. Incomplete involution produces duplication of the portal vein. 2
The portosystemic shunt disrupts the enterohepatic circulation with deranged metabolism of various substances, leading to adverse clinical manifestations. The clinical presentation varies from hypergalactosemia to hyperbilirubinemia to hyperammonimia due to delayed hepatic metabolism of these metabolites as they bypass the liver in the first instance. Pulmonary venous congestion results in hepatopulmonary syndrome, presenting clinically with dyspnea due to pulmonary hypertension. Hepatic encephalopathy may develop in longstanding cases, clinically manifesting with tremors, extrapyramidal symptoms, irritability and altered sensorium. Regenerative nodular hyperplasia of the liver can also result from the liver’s abnormal response to the absent portal flow and can progress to a hepatic tumor in the form of adenoma, hepatoblastoma or hepatocellular carcinoma.3 A female preponderance has been described and associated cardiac and skeletal anomalies, biliary atresia, polysplenia, and hepatic tumors often coexist.4 Prognosis depends on the presence and extent of associated cardiac or hepatic complications.
Early diagnosis and treatment are important to prevent complications. Imaging plays an important part in diagnosing these congenital extrahepatic portosystemic shunts, which have been divided by the Morgan Superina classification of portosystemic anomalies into Type I &Type II shunts.5 Computed tomography with dynamic contrast-enhanced angiography is extremely reliable and better than catheter angiography to demonstrate these congenital extra hepatic portosystemic shunts. The presence or absence of intrahepatic portal vein branches forms the basis of this classification. In Type I CEPS malformations, an end-to-side extrahepatic portosystemic shunt exists, with total diversion of portal mesenteric blood into the IVC, renal or iliac vein, and complete absence of intrahepatic portal vein branches. Type II CEPS malformations constitute incomplete bypass of the liver, and a side-to-side extrahepatic shunt exists between the splanchnic and systemic circulation, with the portal vein being hypoplastic and thin intrahepatic portal vein radicals present in hepatic parenchyma. Type I shunts are further divided into subtype Ia and Ib. In subtype Ia there is no confluence of the SMV and splenic vein, and each drains separately into a systemic, renal, or iliac vein (there is no anatomic portal vein). In subtype Ib, the SMV and splenic vein join to form a portal vein which has a short extrahepatic course with no intrahepatic branches, splanchnic before its abnormal anastomosis, and drainage into a systemic vein, IVC or right atrium. Abernethy malformation belongs to Type Ib CEPS.6
Computed tomography angiography (CTA) helps to differentiate Type I from Type II CEPS shunts and to categorize Type I shunts into subtypes a and b. CTA with arterial and venous phase isolation allows mapping of the course of the shunt and accurate classification. Proper delineation of drainage anatomy is possible with CTA due to its ability to rapidly scan a large volume of tissue and provide multiplanar reconstructions of the vasculature.7 Catheter angiography is no longer preferred, due to the difficulty of performing an invasive vascular puncture in children and its associated complications. Magnetic resonance angiography (MRA), due to its multiplanar capability and superb spatial resolution, is also an excellent technique to visualize these aberrations and confirm hypoplasia or absence of the portal vein.8 Both modalities can detect hepatic nodular hyperplasia and characterize adenoma, hepatoblastoma, or hepatocellular carcinoma.9 Both MRI and CT can detect cerebral complications associated with chronic hepatic failure in Abernethy malformation. These are seen as increased signal intensity in bilateral lentiform nuclei on T1W images (manganese deposition in the globus pallidi) and cerebral atrophy.
Diagnosing CEPS requires exclusion of acquired shunts due to cirrhosis or portal-vein thrombosis. Acquired shunts manifest clinically with portal hypertension and imaging features of splenomegaly, cavernoma, or collaterals at the porta- or intraluminal filling defect in PV. Surgically created portosystemic shunts must also be excluded.10
Knowledge of congenital extrahepatic mesenteric-systemic shunts is important for accurate triage and selection of adequate management options. Determining the type of shunt is important, as CEPS type 1 patients cannot undergo shunt closure; clinical, biochemical, and imaging follow-up with medical management is the only therapeutic option unless a liver transplant is possible. Type II shunts require early closure to prevent hepatic encephalopathy.11 Shunt closure, through either surgery or percutaneous catheterization, results in restoration of intrahepatic portal blood flow in most patients.12
CONCLUSION
Familiarity with congenital aberrant mesenteric drainage is important to accurately diagnose CEPS and Abernethy malformation. Current CT and MR technologies make diagnosis of Abernethy malformation and other CEPS shunts possible. They also demonstrate the spectrum of findings seen in CEPS shunts far better than conventional catheter angiography, obviating the need for the latter in the triage of mesenteric-systemic shunts.
REFERENCES
- Howard E, Davenport M. Congenital extrahepatic portocaval shunts—The Abernethy Malformation. J of Pediatr Surg. 1997; 32(3):494-497.
- Marks C. Developmental basis of the portal venous system. Am J Surg. 1969; 117(5):671-681.
- Akahoshi T, Nishizaki T, Wakasugi K, et al. Portal-systemic encephalopathy due to a congenital extra hepatic Porto systemic shunt: three cases and literature review. Hepatogastroenterology. 2000;47(34): 1113–1116.
- Yoo S, Babyn P, Murray C. Congenital extra hepatic portosystemic shunts. Pediatr Radiol. 2003; 33(9):614-620.
- Morgan G, Superina R. Congenital absence of the portal vein: Two cases and a proposed classification system for Porto Systemic vascular anomalies. J Pediatr Surg. 1994; 29(9):1239-1241.
- Alonso-Gamarra E, Parrón M, Pérez A. Clinical and radiologic manifestations of congenital extra hepatic porto systemic shunts: A comprehensive review. RadioGraphics. 2011; 31(3):707-722.
- Niwa T, Aida N, Tachibana K. Congenital absence of the portal vein: Clinical and radiologic findings. J Comp Assist Tomogr. 2002; 26(5):681-686.
- Konstas A, Digumarthy S, Avery L. Congenital porto systemic shunts: Imaging findings and clinical presentations in 11 patients. Euro J Radiol. 2011; 80(2):175-181.
- Tanaka Y, Takayanagi M, Shiratori Y. Congenital absence of portal vein with multiple hyperplastic nodular lesions in the liver. J Gastroent. 2003; 38(3):288-294.
- Franchi-Abella S, Branchereau S, Lambert V. Complications of congenital porto systemic shunts in children: Therapeutic options and outcomes. J Pediatr Gastroent and Nutr. 2010; 1.
- Lautz T, Tantemsapya N, Rowell E. Management and classification of type II congenital porto systemic shunts. J Pediatr Surg. 2011; 46(2):308-314.
Citation
S M, S W, MA K, UC G. Abernethy malformation. Appl Radiol. 2018;(12):28-30.
December 3, 2018