Acute perineum and scrotum: Cross-sectional imaging findings
Images
The perineum may be easily overlooked during imaging examinations, but it can potentially contain numerous acute conditions in patients presenting with pelvic pain. Appreciation of its anatomy allows radiologists to accurately interpret imaging tests and provide quality care to patients. This article briefly reviews the anatomy of the perineum and scrotum and imaging of various acute conditions occurring in these areas.
Anatomy of perineum
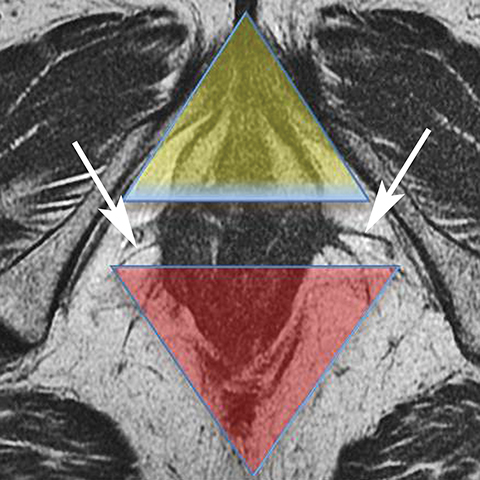
The perineum is a diamond-shaped space that lies inferior to the muscular pelvic floor. The boundaries of the perineum are the pelvic floor superiorly, pubic symphysis anteriorly, coccyx posteriorly, ischial tuberosities laterally, ischiopubic rami anterolaterally, sacrotuberous ligaments posterolaterally, and fascia and skin inferiorly. A line drawn between the ischial tuberosities separates the perineum into an anterior urogenital triangle and posterior anal triangle (Figure 1). This line coincides with the transversus perineus muscle.
The urogenital triangle contains the external openings of the urethra and the vagina. The anal triangle contains the anal canal, ischioanal fossa, and the pudendal canal. 1 The ischioanal fossa is inferior to the pelvic diaphragm, which comprises the levator ani and coccygeus muscles.
The perineum contains superficial and deep pouches. The superficial pouch is enclosed between a membranous layer of fascia inferiorly (also referred to as Colle’s fascia) and the perineal membrane superiorly (also referred to as the inferior fascia of the urogenital diaphragm). The superficial pouch has structures common to both males and females, including the ischiocavernosus, bulbospongiosus, superficial transversus perinei muscles, the pudendal vessels, and pudendal nerves. Unique structures in the male include the root of the scrotum, bulb of the penis, and the penile urethra. Unique structures in females are the greater vestibular glands (Bartholin’s glands), Skene glands, vaginal vestibule, clitoris, and vulva.
The deep perineal pouch contains the urogenital diaphragm, its investing fascia, and structures that pass through it. The internal pudendal artery passes through and is the primary blood supply to the perineum. The pudendal nerve, arising from the S2-4 sacral nerves, accompanies the artery. Men possess the membranous urethra, bulbourethral glands (Cowper’s glands), and dorsal artery and nerve of the penis. Women have corresponding structures, but are notable for lack of Cowper’s glands, the presence of the inferior vagina at the level of the introitus, and the piercing of the sphincter urethrae by the inferior two-thirds of the urethra. The vulva (external genitalia) is also part of the perineum.
The perineum is supported by the pelvic and urogenital diaphragms and the perineal body; the perineal body (also referred to as the central tendon) is a pyramidal fibromuscular structure located at the junction of the urogenital and anal triangles, resulting from the convergence of several pelvic muscles.
Vascular conditions
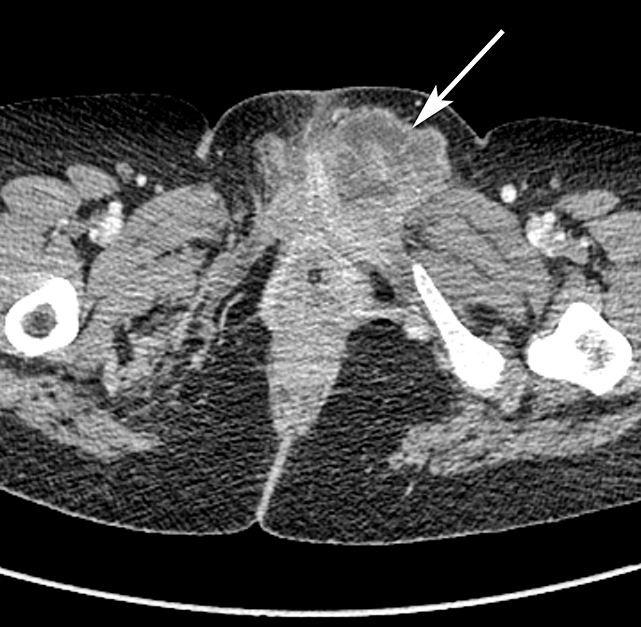
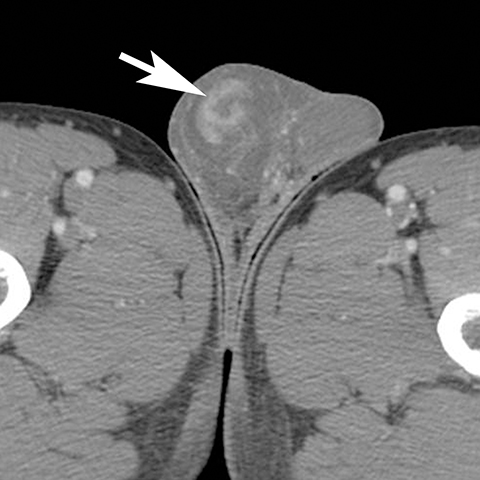
The majority of nonobstetric, accidental perineal trauma is due to blunt force in the form of straddle injuries. In women, the vulva is the most external aspect of the perineum and most blunt force injuries are confined to the labia.2 Typical CT findings following blunt trauma consist of a heterogeneous hematoma within the subcutaneous tissues or within the tissue planes of the labia (Figure 2). The majority of these injuries are treated conservatively and without imaging follow-up. Non-straddle blunt and penetrating injuries are more complex and are more likely to require surgery. Postcontrast CT is useful to evaluate for active hemorrhage or traumatic pseudoaneurysm.3 Active hemorrhage appears as an amorphous blush of contrast that is initially isodense to adjacent vessels, but that decreases in density, but enlarges over time, while a pseudoaneurysm is typically rounded or oval and follows the contrast density of the vessel from which it originates. Vascular injuries in the perineum typically affect branches of the pudendal artery which originates from the internal iliac artery (Figure 3).
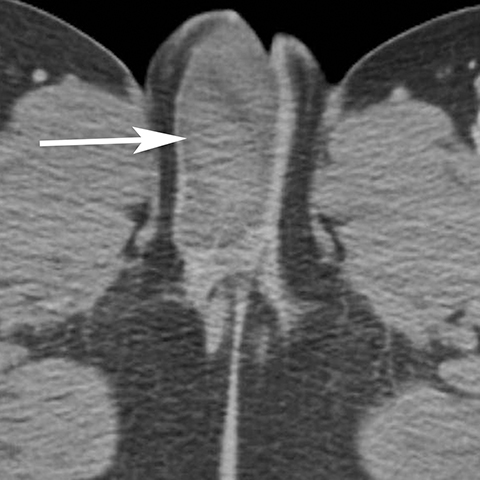
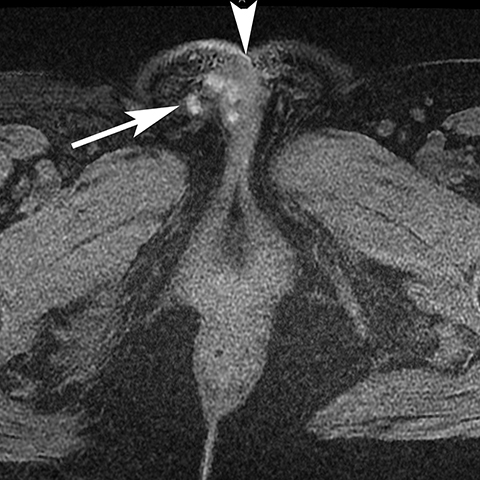
Vulvar thrombophlebitis is a rare phenomenon that can occur in established varicosities during pregnancy or the post-partum period.4 Alternatively, tamoxifen has been reported to cause idiopathic thromboembolism and has been reported to involve the superficial veins of the lower extremity and perineum (Figure 4).5,6
Infectious conditions
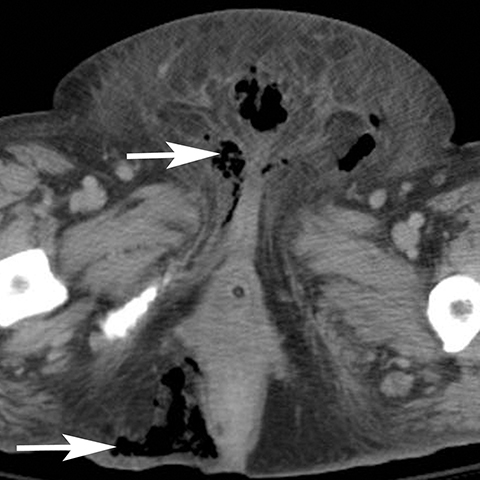
Infection can affect any perineal structure and imaging often plays a central role in determining the severity and response to treatment. Trauma, diabetes, pregnancy, and obesity are risk factors that increase the probability of infection of the vulva. Pregnancy can result in vulvar edema, lymphostasis, bacterial colonization which lead to cellulitis, abscess, or necrotizing fasciitis.7 Post contrast CT or MR shows thickening and hyperemia of the skin and infiltration of subcutaneous tissues (Figure 5). T2-weighted sequences show increased signal of the inflamed tissue. Cellulitis usually responds to antibiotic treatment and does not require follow up imaging. Uncommonly, cellulitis can progress to vulvar abscess for which surgical drainage may be needed.8 Vulvar abscess should be suspected on imaging when an irregular, rim-enhancing collection is identified on postcontrast CT or MRI. Severe infections can progress to necrotizing fasciitis which can be rapidly fatal if emergent debridement is not performed. Diabetics are especially susceptible to Fournier’s gangrene and involvement of the perineum is associated with high mortality (Figure 6).9,10 CT findings include soft tissue thickening and inflammation, abscess, and subcutaneous emphysema.11
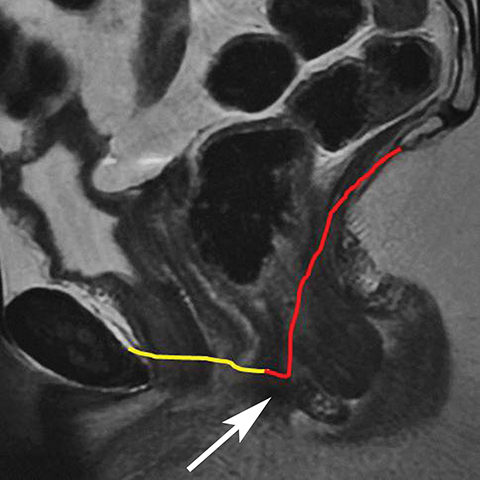
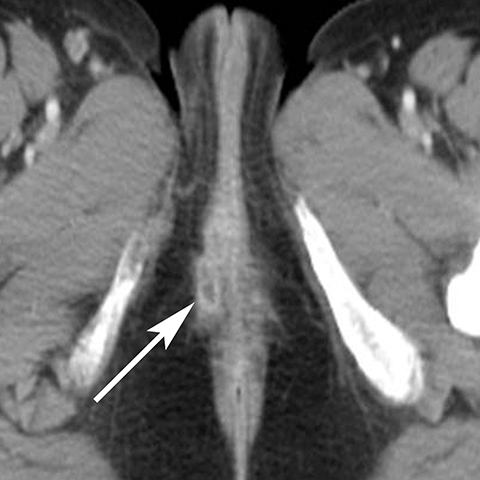
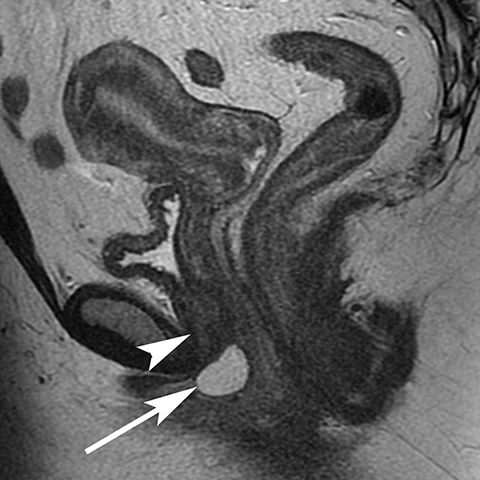
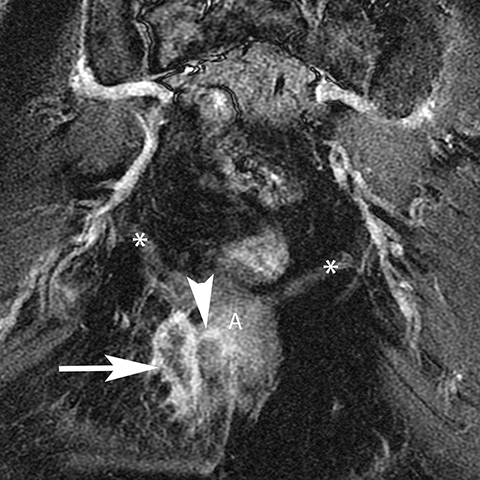
The Bartholin (greater vestibular) and Skene (periurethral) glands are another source of perineal infection. Bartholin glands are located in the superficial perineal pouch, with ducts opening into the posterolateral aspects of the vaginal vestibule. Ductal obstruction can lead to cyst formation and subsequent infection/abscess. On CT, Bartholin gland cysts appear as well marginated, hypoattenuating structures along the posterolateral wall of the vagina; MR typically shows low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. On sagittal view, they arise below the perineal membrane and the inferior border of the pubic symphysis, in contrast to the more superiorly located Gardner duct cysts. Infected Bartholin glands may show more heterogeneous contents due to variable amounts of cellular, proteinaceous, and hemorrhagic debris. Additionally, irregular rim enhancement may signify abscess development (Figure 7).12
Skene glands are located along the lateral aspect of the distal two-thirds of the urethra and provide urethral lubrication. Obstruction of the duct that drains into the urethral lumen can lead to development of Skene gland cysts or abscesses. With the exception of location, the imaging characteristics for Skene cysts and abscess are similar to those described for the Bartholin glands. However, it should be noted that both Skene and Bartholin gland cysts can harbor infection despite having a simple appearance on imaging; if the patient is having pain at the site of a ‘cyst’ noted on imaging, the collection should be considered to be infected (Figure 8).13 Skene gland cysts and abscesses are precursors to urethral diverticula; rupture into the lumen of the urethra results in a urethral diverticulum.
Perianal conditions
Perianal fistulas and abscesses may be secondary to Crohn’s disease, trauma, malignancy, and radiation therapy. MR is the imaging modality of choice for the detection and characterization of fistulas. Most perianal fistulas extend within the intersphincteric space to the skin surface, while a minority pierce both layers of the anal sphincter muscle and traverse the ischioanal space.14,15 Differentiating between inter- and transsphincteric fistulas is important for surgical planning and is typically described according to the St. James classification.14,16 Fistulas appear as linear tracts of low T1 signal intensity and high T2 signal intensity (Figure 9). Active fistulas will show mural enhancement and will be fluid filled while chronic, healing fistulas will enhance more homogeneously and will lack intraluminal fluid. Anovaginal fistulas may be due to trauma, surgery, or radiation therapy. MR will show a thin channel connecting the posterior wall with the anterior wall of the vagina (Figure 10).
Scrotal anatomy
The scrotum consists of 2 sacs divided by a midline raphe; each hemiscrotum contains a testis (which typically measures approximately 5 × 3 × 2cm), epididymis, spermatic cord, and fascial covering.17 Each testis is invested by the fibrous tunica albuginea. The scrotal wall consists of the following layers (from superficial to deep): skin, superficial fascia, dartos muscle, external spermatic fascia, cremasteric fascia, and internal spermatic fascia. The tunica vaginalis, covering the tunica albuginea, is double-layered and has an outer parietal layer lining the inner scrotal wall to form the mediastinum.18 Seminiferous tubules open from the mediastinum into the rete testis and drain into the epididymis. The spermatic cord begins at the deep inguinal ring and descends vertically into the scrotum; the spermatic cord contains the vas deferens, testicular artery, cremasteric artery, deferential artery, pampiniform plexuses, genitofemoral nerve, and lymphatic vessels.18,19
Ultrasound (US), typically performed with a high frequency (7-15 MHz), linear-array transducer, is the imaging modality of choice for examination of the acute scrotum. The application of Doppler US is essential when evaluating various acute conditions.
Scrotal inflammation and infection
Epididymitis and epididymo-orchitis are common causes of acute scrotal pain. In young adults, the condition is typically secondary to sexually transmitted organisms, such as Neisseria gonorrhoeae and Chlamydia trachomatis. However, in prepubertal boys and in men older than 35 years, the most common causative organisms are Escherichia coli and Proteus mirabilis.20 Additional rare etiologies include various infections (tuberculosis, mumps), medications (amiodarone), and sarcoidosis.21
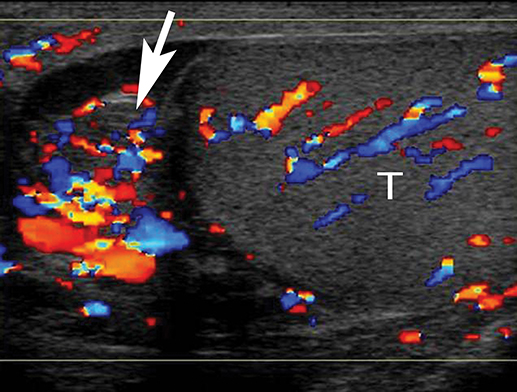
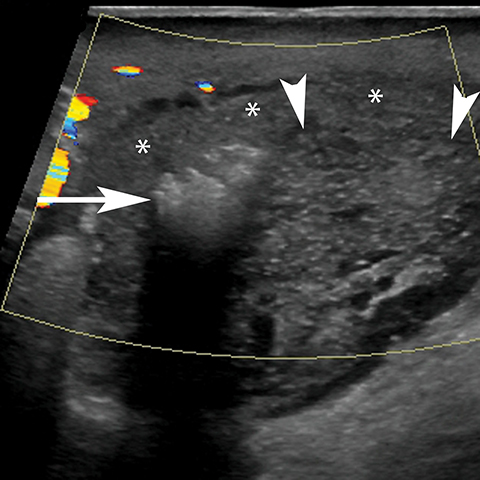
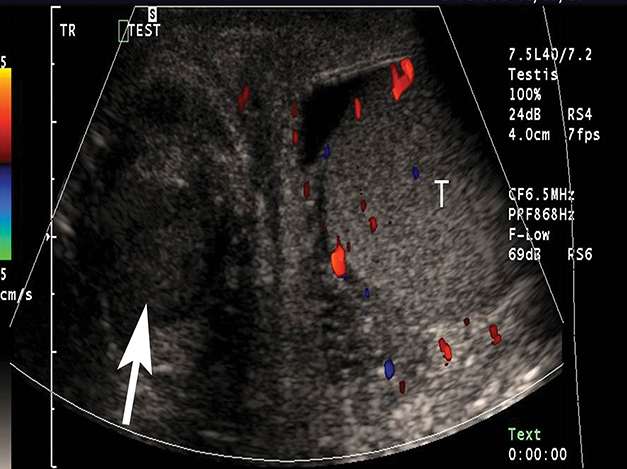
At gray-scale US, imaging findings generally consist of an enlarged, hypoechoic epididymis; occasionally, areas of hyperechogenicity may reflect hemorrhage. Application of color Doppler shows diffuse hyperemia (Figure 11) compared to the contralateral epididymis; spectral Doppler interrogation will demonstrate a high-flow, low-resistance pattern often resulting in a resistive index below 0.5.22 In up to approximately 40% of cases, orchitis develops due to contiguous spread of infection; US findings include an enlarged, heterogeneous, and hyperemic testis. In some cases, ochitis may be focal and manifest as several hypoechoic lesions that can be difficult to differentiate from a neoplastic process by imaging alone. Additional associated findings include scrotal wall thickening and hydrocele. If untreated, epididymo-orchitis can progress to abscess, pyocele, or infarction.23
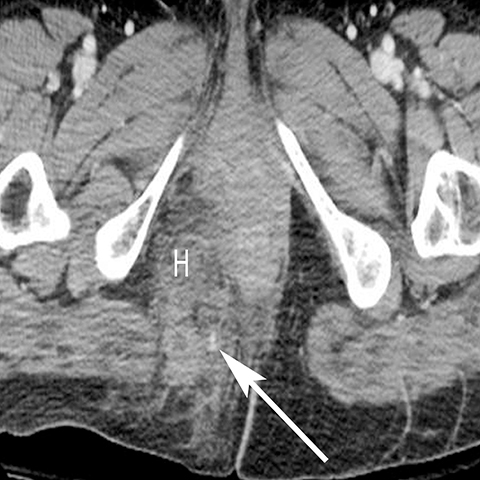
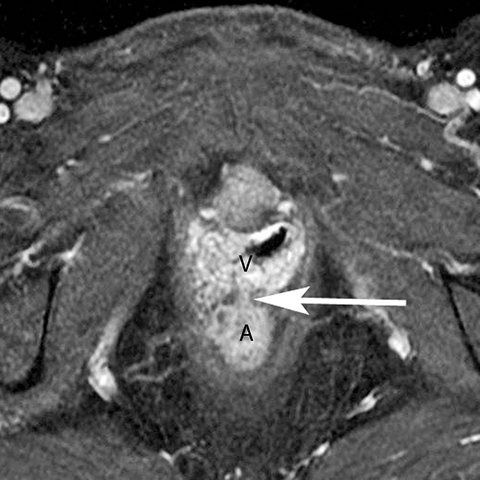
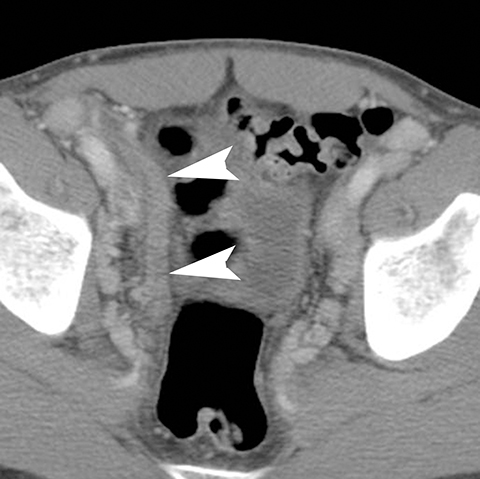
In addition to the spread of infection and inflammation to the testis, the vas deferens may also become secondarily infected by epididymitis. The retrograde spread along the vas deferens can result in pelvic pain and thickening of the spermatic cord in the inguinal canal and vas deferens as it transits the lateral aspect of the pelvis. US may show nonspecific thickening and hyperemia of the spermatic cord in the inguinal canal. During postcontrast CT, the inflamed vas deferens will appear as a thickened, hyperemia cord-like structure running obliquely along the pelvic sidewall medial to the external iliac vessels (Figure 12).24
In addition to severe or untreated epididymo-orchitis, a scrotal abscess can also result from trauma or infarction. Scrotal abscesses can be intra- or paratesticular; regardless of location, patients with an abscess may present with scrotal pain, erythema, induration, fever, or leukocytosis. On US, scrotal abscesses generally have thick, irregular walls and contain debris appearing as low-intermediate internal echoes (Figure 13). While abscesses lack internal color flow, a hyperemic rim may be occasionally observed.25 Abscesses that contain gas locules will show brightly echogenic reflectors that result in posterior shadowing which may sometimes be heterogeneous (‘dirty’ shadowing). Postcontrast CT or MR is less commonly performed for the evaluation of scrotal abscess, but will can show a rim-enhancing fluid collection within the testis. Follow-up US after treatment of epididymo-orchitis or abscess is typically recommended to assure complete resolution.
Scrotal trauma
The majority of traumatic scrotal injuries are due to blunt trauma and include hematoma/hematocele, rupture, and fracture. US has been reported to have 100% sensitivity for detection of scrotal injuries and 80% specificity for tunica albuginea fractures.26 Hematomas can occur in the scrotal wall, epididymis, testis, or tunica vaginalis (hematocele). Hematomas can have variable echogenicity due to the age of the blood products; acute hematomas are echogenic but eventually lyse to become complex, septated, cystic collections as they regress and evolve. While hematomas are devoid of color Doppler flow, adjacent tissues may be mildy hyperemic.23,27 Intra-testicular hematomas may lead to necrosis or infection in up to 40% of patients.
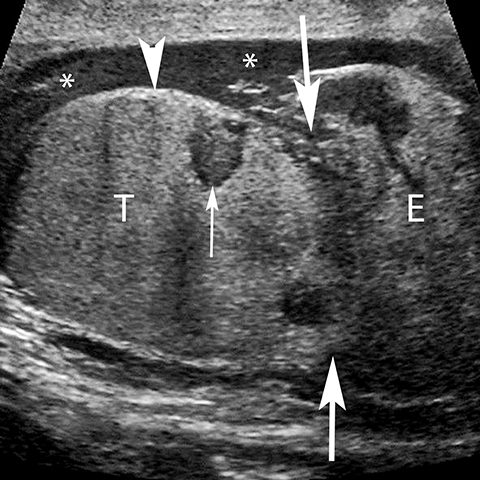
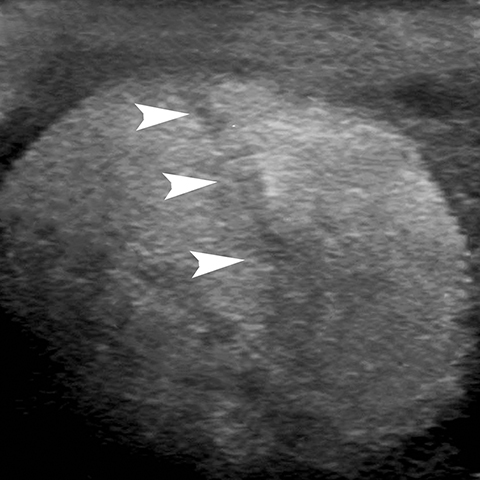
Rupture of the testis is a surgical emergency since greater than 80% of testes can be salvaged if intervention is undertaken within the first 72 hours of the traumatic event.28 The US findings of contour abnormality of the testis, disruption of the tunica albuginea, and heterogeneous echotexture are considered to be sensitive and specific for the diagnosis of testis rupture (Figure 14). Identification of a fracture line through the testis is uncommon, but manifests as an avascular linear or curvilinear hypoechoic defect on US (Figure 15). The presence of a fracture line does not necessarily imply rupture of the testis. The presence of a testis fracture, rupture or of a hematocele are indications for surgical exploration. Trauma to the testis can also lead to complications such as epididymitis, atrophy, and infertility.
Vascular conditions
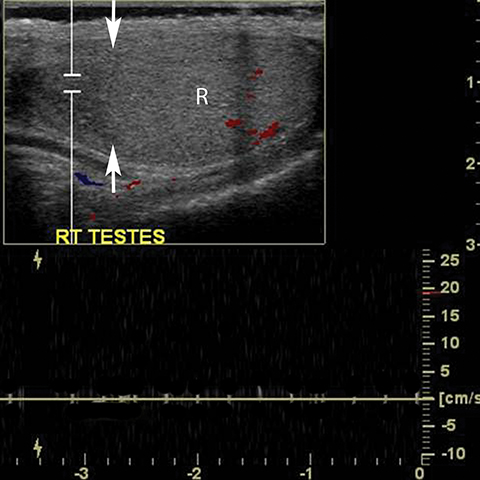
Testicular torsion most commonly affects 12-18 year olds but can occur at any age.23 Except for neonates, torsion is intravaginal due to a long, narrow mesentery or the ‘bell clapper’ deformity in which the tunica vaginalis completely encircles the epididymis, distal spermatic cord, and testis thereby preventing the normal attachment of the testis to the scrotal wall. Patients present with acute scrotal pain often accompanied by nausea and vomiting. Unlike epididymitis, the cremasteric reflex is absent in torsion and pain is not relieved by elevation of the scrotum.29 Testicular torsion initially results in compromise of venous flow, followed by a reduction in arterial flow; studies have suggested that approximately 720° of twisting of the spermatic cord is needed before the artery is occluded. 30 The surgical salvage rate is inversely proportional to the degree of spermatic cord twisting and the duration of ischemia, ranging from nearly 100% success within the first 6 hours and only 20% success at 12-24 hours after the onset of symptoms. 31 The absence of color Doppler US has reported sensitivity of 86%, specificity of 100%, accuracy of 97% (Figure 16).32 Power Doppler allows more sensitive evaluation for slow flow.33 Findings at gray scale imaging are nonspecific as they vary with the duration of torsion; gray scale evaluation can be normal if scanning is performed approximately less than 4 hours after the onset of symptoms.32 After 4-6 hours, parenchymal hypoechogencity becomes apparent followed by parenchymal heterogeneity at approximately 24 hours due to increasing edema, hemorrhage, and development of necrosis (Figure 16).32 Ancillary findings include hydrocele and scrotal skin thickening.
In addition to the testis, testicular appendages can also undergo torsion, typically occurring in patients 7-14 years of age. The main US finding is a hyperechoic nodule with central hypoechogenicity adjacent to the testis or epididymis. Doppler US plays less of a role since normal appendages do not show internal flow.
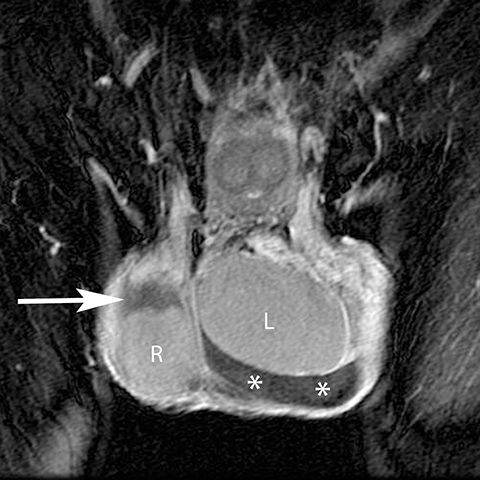
While prolonged testicular ischemia due to torsion can lead to global testicular infarction, segmental infarction may result from embolic sources and focal vascular injury (such as during inguinal herniorraphy). Segmental infarctions manifest as a geographic region of hypoechogenicity that is devoid of Doppler flow or enhancement at MR (Figure 17).
Conclusion
The perineum and scrotum are subject to various acute conditions. Familiarity with the anatomy and common acute diagnoses is important for evaluating the perineum and scrotum in patients presenting with pelvic pain or injuries. Failure to include the perineum and scrotum during cross-sectional imaging may lead to missed pathology.
References
- Stoker J. Anorectal and pelvic floor anatomy. Best Pract Res Clin Gastroenterol. 2009;23(4):463-475.
- Iqbal CW, Jrebi NY, Zielinski MD, et al. Patterns of accidental genital trauma in young girls and indications for operative management. J Ped Surg. 2010;45(5):930-933.
- Kunishima K, Takao H, Kato N, et al. Transarterial embolization of a nonpuerperal traumatic vulvar hematoma. Radiat Med. 2008;26(3):168-170.
- Veltman LL, Ostergard DR. Thrombosis of vulvar varicosities during pregnancy. Obstet Gynecol. 1972;39(1):55-56.
- Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442-4449.
- Hosseinzadeh K, Heller MT, Houshmand G. Imaging of the female perineum in adults. Radiographics. 2012;32(4):E129-168.
- Kulas T, Habek D, Hrgovic Z. Massive labia minor hypertrophy following vulvar edema and abscess in pregnancy--case report. Z Geburtshilfe Neonatol. 2009;213(5):207-209.
- Thurman AR, Satterfield TM, Soper DE. Methicillin-resistant Staphylococcus aureus as a common cause of vulvar abscesses. Obstet Gynecol. 2008;112:538-544.
- Addison WA, Livengood CH, 3rd, Hill GB, et al. Necrotizing fasciitis of vulvar origin in diabetic patients. Obstet Gynecol. 1984;63(4):473-479.
- Roberts DB. Necrotizing fasciitis of the vulva. Am J Obstet Gynecol. 1987;157(3):568-571.
- Levenson RB, Singh AK, Novelline RA. Fournier gangrene: role of imaging. Radiographics. 2008;28(2):519-528.
- Siegelman ES, Outwater EK, Banner MP, et al. High-resolution MR imaging of the vagina. Radiographics. 1997;17(5):1183-1203.
- Eilber KS, Raz S. Benign cystic lesions of the vagina: a literature review. J Urol. 2003;170(3):717-722.
- Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20(3):623-635; discussion 635-627.
- Laniado M, Makowiec F, Dammann F, et al. Perianal complications of Crohn disease: MR imaging findings. Eur Radiol. 1997;7(7):1035-1042.
- Jehle EC, Haehnel T, Starlinger MJ, et al. Level of the anastomosis does not influence functional outcome after anterior rectal resection for rectal cancer. Am J Surg. 1995;169(1):147-152; discussion 152-143.
- Woodward PJ, Schwab CM, Sesterhenn IA. From the archives of the AFIP: extratesticular scrotal masses: radiologic-pathologic correlation. Radiographics. 2003;23(1):215-240.
- Dogra VS, Gottlieb RH, Oka M, et al. Sonography of the scrotum. Radiology. 2003;227(1):18-36.
- Langer JE. Ultrasound of the scrotum. Semin Roentgenol. 1993;28(1):5-18.
- Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35(1):101-108; vii.
- Mirochnik B, Bhargava P, Dighe MK, et al. Ultrasound evaluation of scrotal pathology. Radiol Clin North Am. 2012;50(2):317-332, vi.
- Lerner RM, Mevorach RA, Hulbert WC, et al. Color Doppler US in the evaluation of acute scrotal disease. Radiology. 1990;176(2):355-358.
- Dogra V, Bhatt S. Acute painful scrotum. Radiol Clin North Am. 2004;42(2):349-363.
- Eddy K, Piercy GB, Eddy R. Vasitis: clinical and ultrasound confusion with inguinal hernia clarified by computed tomography. Can Urol Assoc J. 2011;5(4):E74-76.
- Dogra VS, Gottlieb RH, Rubens DJ, et al. Benign intratesticular cystic lesions: US features. Radiographics. 2001;21 Spec No:S273-281.
- Sasso F, Gulino G, Di Pinto A, et al. [Correlation between ultrasonography imaging and surgical findings in scrotal trauma]. Arch Ital Urol Androl. 1995;67(2):159-162.
- Gordon LM, Stein SM, Ralls PW. Traumatic epididymitis: evaluation with color Doppler sonography. AJR Am J Roentgenol. 1996;166(6):1323-1325.
- Bhandary P, Abbitt PL, Watson L. Ultrasound diagnosis of traumatic testicular rupture. J Clin Ultrasound. 1992;20(5):346-348.
- Noske HD, Kraus SW, Altinkilic BM, et al. Historical milestones regarding torsion of the scrotal organs. J Urol. 1998;159(1):13-16.
- Herbener TE. Ultrasound in the assessment of the acute scrotum. J Clin Ultrasound. 1996;24(8): 405-421.
- Patriquin HB, Yazbeck S, Trinh B, et al. Testicular torsion in infants and children: diagnosis with Doppler sonography. Radiology. 1993;188(3):781-785.
- Burks DD, Markey BJ, Burkhard TK, et al. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815-821.
- Barth RA, Shortliffe LD. Normal pediatric testis: comparison of power Doppler and color Doppler US in the detection of blood flow. Radiology. 1997;204(2):389-393.
Citation
MT H, A P. Acute perineum and scrotum: Cross-sectional imaging findings. Appl Radiol. 2016;(3):18-23.
March 2, 2016