MRI of endometriosis: A comprehensive review
By Samreen N, Bookwalter CA, Sheedy SP, VanBuren WA, Burnett TL, Feldman M, Menias C
Endometriosis is defined as non- neoplastic endometrial glands and stroma residing outside of the uterine cavity and myometrium. This ectopic endometrium responds to hormonal stimulation, causing various degrees of cyclic hemorrhage, which results in inflammation, fibrosis, and adhesion formation in the surrounding tissues. It is often a debilitating disease due to pain and infertility, especially in young women. Endometriosis most commonly affects women of childbearing age, with a mean age at presentation of 25-29 years. The estimated prevalence of endometriosis is 5-10%, including both symptomatic and asymptomatic females.1
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Approximately 5% of endometriosis is seen in postmenopausal women.2 There is no racial predilection. There is an increased prevalence in relatives of affected individuals, suggesting a genetic component.
The risk factors for endometriosis are thought to be related to increased exposure to menstruation. These include early age of menarche, short menstrual cycle, long duration of menstrual flow, nulliparity, and positive family history. Endometriosis in girls <17 years of age may be seen with obstructive Mullerian duct anomalies of the cervix and vagina. In postmenopausal women, exogenous estrogen replacement therapy is hypothesized to be a causative factor, however cases without exogenous estrogen exposure are also described.2
Females commonly present with chronic pelvic pain, dyspareunia, and/or infertility. Other symptoms depend on location of endometriosis and organs involved; however, symptoms do not necessarily correlate with extent or severity of disease. It is seen in up to 90% of females with chronic pelvic pain and 20-50% of females with infertility. Radiologist familiarity with the various imaging appearances of endometriosis may permit earlier diagnosis, reduce treatment delays, and minimize the financial impact of the disease.
Pathogenesis
Multiple theories regarding the pathogenesis of endometriosis have been proposed. Currently, the most widely accepted is the retrograde menstruation theory which hypothesizes that retrograde menstruation allows endometrial tissue to reflux through the fallopian tubes and implant on peritoneal surfaces or pelvic organs where it can continue to grow with hormonal cycles. Alternatively, the metaplastic theory suggests that existing peritoneal cells differentiate into functioning endometrial cells and is based on embryological development since both peritoneal and endometrial tissue are derived from coelomic wall epithelium. Finally, the induction theory suggests a combination of the two above mentioned theories, proposing that shed endometrium releases substances that allow undifferentiated mesenchyme to differentiate into endometriotic tissue.3
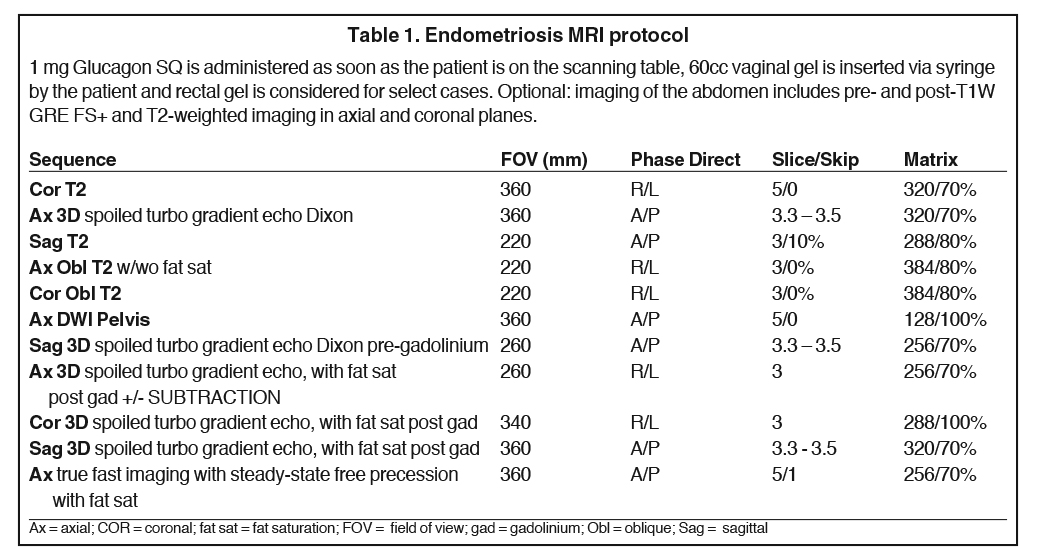
MRI protocol
A dedicated MRI protocol is essential for identification of disease and surgical planning. MRI imaging at 3 Tesla is preferred due to superior resolution. Specific sequences with and without fat saturation are helpful in differentiating cystic teratomas from endometriomas. Diffusion-weighted sequences can be helpful in identifying endometriotic deposits that restrict diffusion secondary to blood products, and can be used for the assessment of malignant transformation. Sagittal T1- and T2-weighted imaging are very helpful for identifying disease in the anterior and posterior cul-de-sacs, which may be overlooked on axial or coronal imaging. Administration of intravenous contrast is important, as areas of mural nodularity or solid components may exist within an ovarian lesion, and is essential to differentiate endometriomas from other cystic neoplasms. Subtraction of pre- and postcontrast T1-weighted images is also often helpful, as the intrinsic T1-weighted hyperintensity within the endometrioma can make it difficult to visually assess for contrast enhancement. Anti-peristaltic agents help reduce motion artifacts on the studies and improve visualization of bowel lesions. Rectal and vaginal gel help optimize visualization of endometriosis deposits on the vaginal and rectal wall. At Mayo Clinic we routinely use vaginal gel and rectal gel for troubleshooting or for patients who are suspected of having rectosigmoid involvement. Table 1 details the endometriosis imaging protocol used at Mayo Clinic.
Location and histologic types of endometriosis
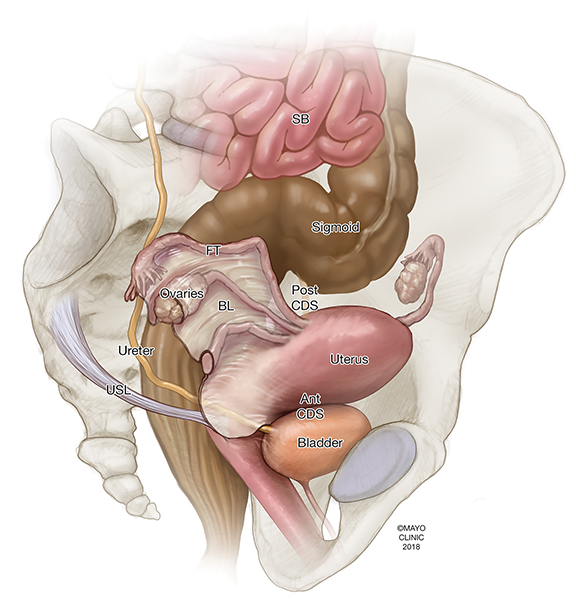
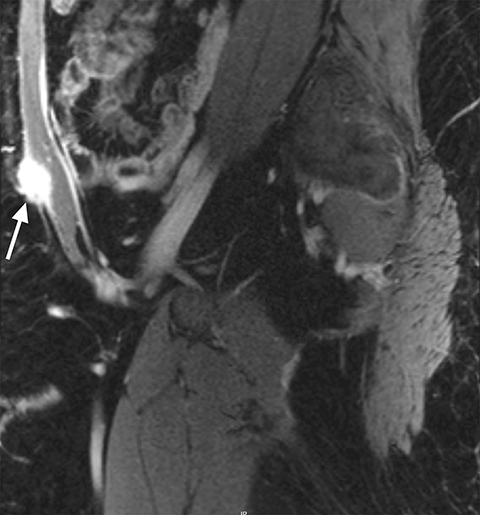
Endometriosis can be intraperitoneal or extraperitoneal. In the pelvis, endometriosis commonly involves the peritoneum (anterior and posterior cul-de-sacs, pelvic side walls and ovarian fossa), uterosacral ligaments, ovaries, fallopian tubes, and uterus. Other structures less commonly involved include the rectovaginal septum, rectum, sigmoid colon, appendix, ureters, and bladder. Additional extra-pelvic endometriosis is uncommon but can involve the diaphragm, cecum, small and large bowel, abdominal wall, and other abdominal organs.4 Other areas to assess include the inguinal canals, sciatic and sacral nerves, peri-hepatic region and pleura (Figure 1).
There are three forms of intraperitoneal pelvic endometriosis. These include superficial peritoneal lesions (ie, noninvasive implants), endometriomas (ie, cystic endometriosis), and deep (or solid and/or cystic) infiltrating endometriosis (DIE).
Superficial endometriosis
Superficial peritoneal implants are hemorrhagic and nonhemorrhagic deposits on the surface of pelvic organs or the peritoneum, favoring the cul-de-sacs and adnexae.4 They start as tiny “powder burns,” which appear blue or black on laparoscopy, but can also be white or red, depending on the degree of fibrosis, scarring, and hemorrhage. They can grow with monthly bleeding and generate plaque-like reactive fibrosis. Small nonhemorrhagic foci of superficial endometriosis are often not detectable with MRI or ultrasound due to their small size, whereas they are easily identified at laparoscopy. Superficial peritoneal implants are hemorrhagic and nonhemorrhagic deposits on the surface of pelvic organs or the peritoneum, favoring the cul-de-sacs and adnexae.4 They are classically described as tiny “powder burns” which appear blue or black on laparoscopy, but can be white, clear, or red, depending on the degree of fibrosis, scarring, and hemorrhage. They may appear as plaque-like reactive fibrosis. Superficial endometriosis is often not detectable with MRI or ultrasound.
Endometriomas
Endometriomas (or cystic ovarian endometriosis) are pseudocysts formed by cyclic hemorrhage into an endometrial implant, causing accumulation of blood products which become trapped by surrounding tissues. As free water in the cyst is resorbed, the concentration of iron increases along with the viscosity of the cyst contents. They are given the name “chocolate cysts” due to their classic appearance at surgery where they appear to contain dark viscous material surrounded by a fibrous wall of variable thickness.5
The ovaries are the most common location for endometriomas. They are often bilateral and multifocal. Ultrasound is the imaging modality of choice for diagnosis of endometriomas due to wide availability and low cost. MRI is used predominantly for problem solving and, in many institutions, for staging of more advance disease. MRI has a 90% sensitivity, 98% specificity, and 96% accuracy for diagnosis of endometriomas5 compared to ultrasound which has a sensitivity of 78% and specificity of 88%.6
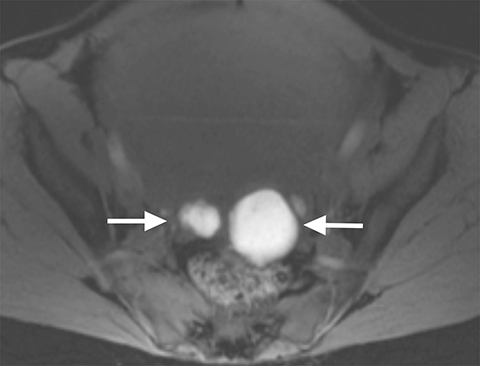
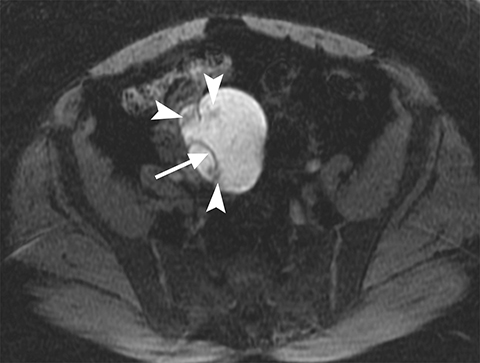
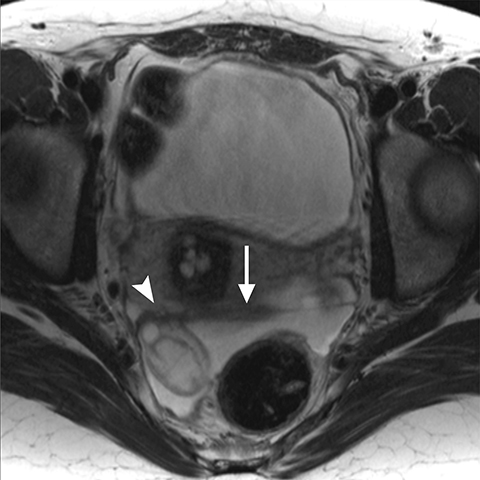
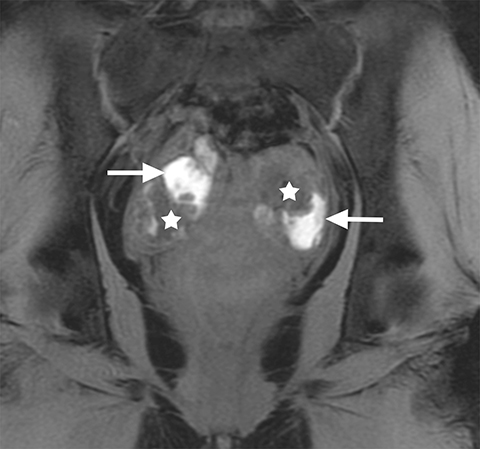
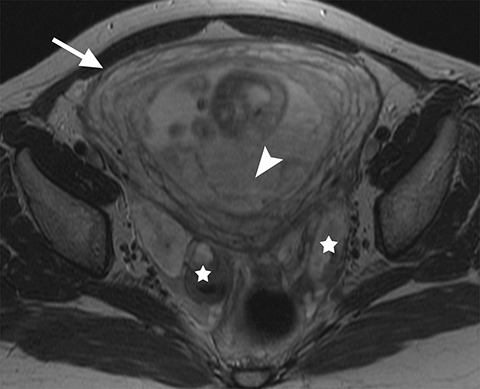
On MRI, ovarian endometriomas have a characteristic homogeneous T1W hyperintensity and a relatively low T2W signal intensity. There can be heterogeneity to the T2W hypointensity, called T2W “shading”, caused by blood products in various stages of degradation from multiple episodes of bleeding. A more specific sign in the diagnosis of ovarian endometriomas is the “T2 dark spot sign,” defined as discrete markedly hypointense foci within the cyst on T2-weighted images, with or without T2 shading. In distinguishing ovarian endometriomas from non-endometrioma hemorrhagic cystic lesions, a study has shown T2 shading to have a 93% sensitivity, 45% specificity, 72% positive predictive value (PPV) and 81% negative predictive value (NPV), while T2 dark spots had a 93% sensitivity, 45% specificity, 72% PPV and 81% NPV (Figure 2).7
On diffusion sequences, the cystic component of ovarian endometriomas can show diffusion restriction with low apparent diffusion coefficient (ADC) values due to their hemorrhagic/proteinaceous contents. Therefore, diffusion sequences are not helpful in differentiating endometriomas from cystic ovarian neoplasms unless assessment is directed at a solid, enhancing nodule or endometriotic deposit.
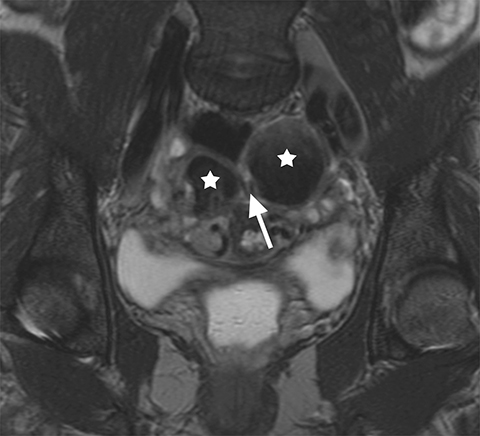
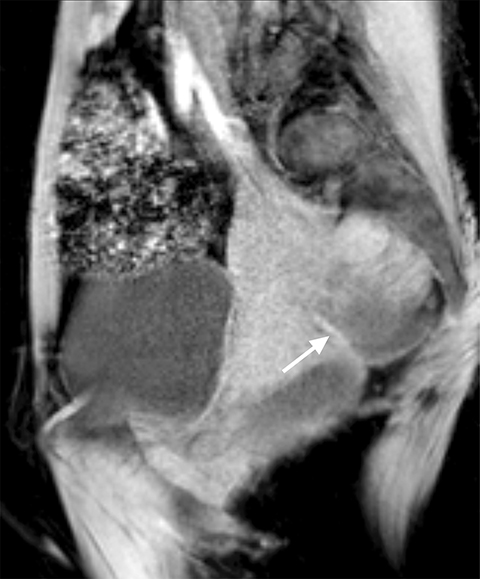
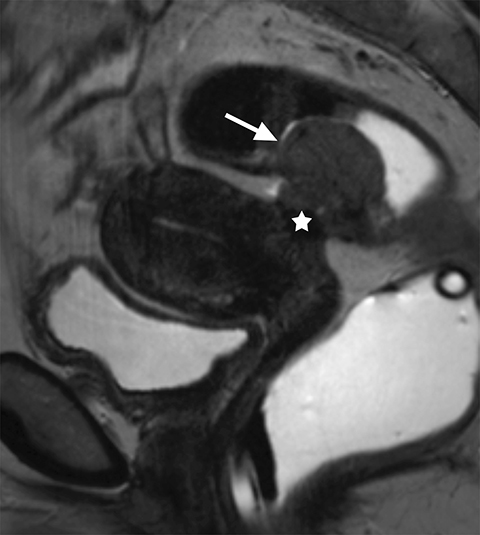
The main differential considerations of an ovarian endometrioma include other cystic adnexal lesions such as hemorrhagic cysts, cystic teratomas and cystic ovarian neoplasms. A study by Outwater et al showed that endometriomas have a higher T1W and a lower T2W signal intensity in comparison with hemorrhagic cysts.8 Bilateral and multifocal lesions and thicker, low T2 signal intensity fibrotic rims are also noted to be more suggestive of an endometrioma.9 Fat suppression sequences are helpful in differentiating cystic ovarian teratomas from endometriomas, as macroscopic fat within the cystic teratomas will suppress with chemical fat suppression techniques. However, it should be noted that STIR sequences use frequency selective fat suppression techniques, and since the frequency of fat can be similar to the frequency of hemorrhagic/proteinaceous contents within the endometrioma, an ovarian endometrioma can show falsely suppressed signal with STIR sequences and may be confused with a cystic teratoma. Lastly, it is important to assess for enhancing mural/solid nodules within a cystic adnexal lesion as this may suggest malignancy, but can also be seen in benign endometriomas (Figure 3).10
Deep infiltrating endometriosis (DIE)
DIE is defined as invasion of endometrial glands and stroma ≥5 mm beneath the peritoneal surface.9 The endometrial glands and stroma infiltrate into adjacent fibromuscular tissue and cause smooth muscle proliferation and fibrotic reaction, resulting in solid nodule formation. Dedicated ultrasound exam protocols for DIE have a reported sensitivity of 78.5% and pelvic MRI has a reported sensitivity of 90.3% for the detection of DIE. On MRI, these lesions are typically low to intermediate signal intensity on T1-weighted sequences, and they may or may not have punctate foci of high T1W signal representing the hemorrhagic foci surrounded by the fibrous tissue. However, this form of endometriosis usually has less T1W hyperintensity because cyclical bleeding within the endometrial glands is minimized by surrounding smooth muscle hypertrophy and fibrosis. As a result, DIE may appear both T1W and T2W hypointense. Occasionally small foci of T2W hyperintensity representing the ectopic endometrial glands can be seen.9 This solid type of endometriosis may be harder to identify on MRI, however, postcontrast, these lesions demonstrate delayed enhancement similar to fibrosis.
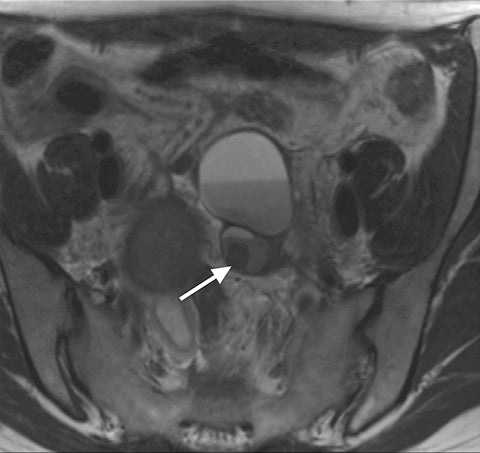
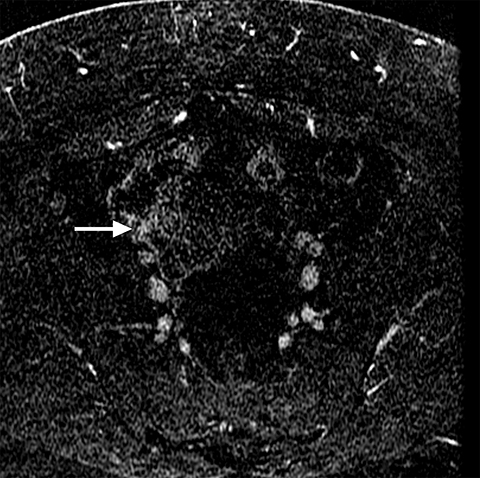
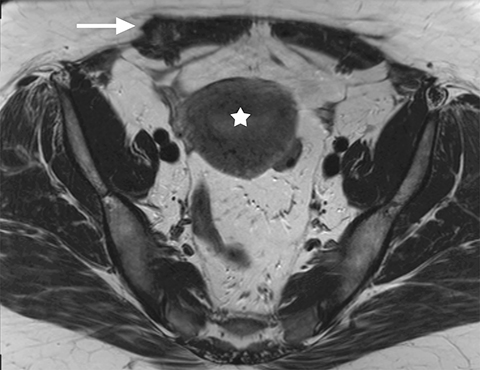
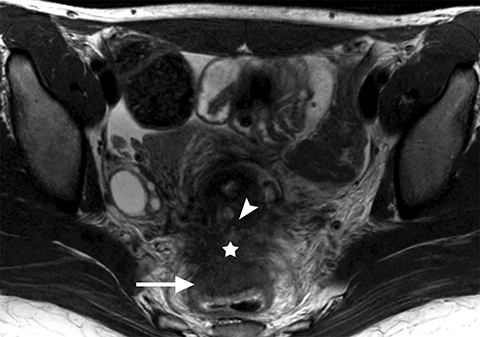
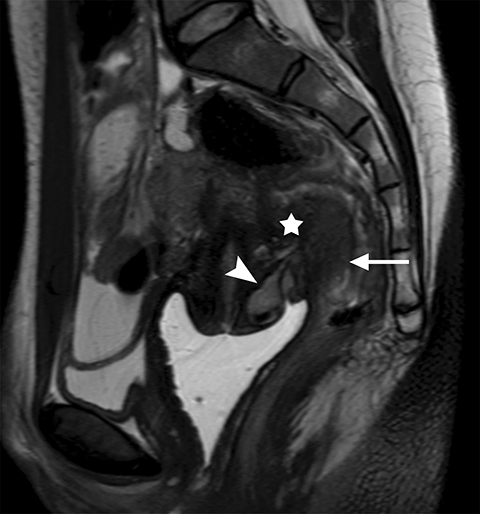
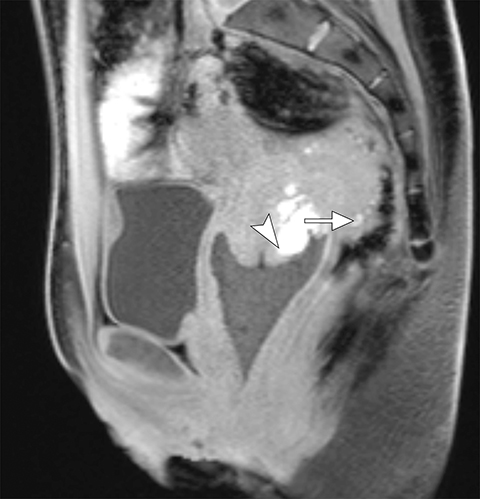
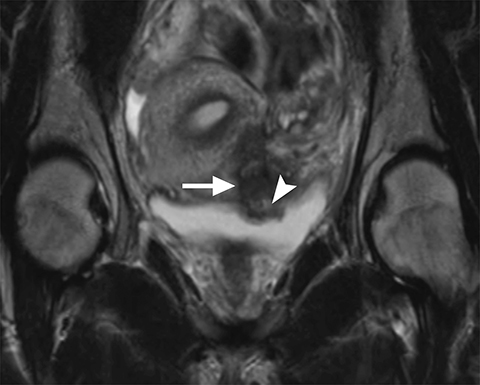
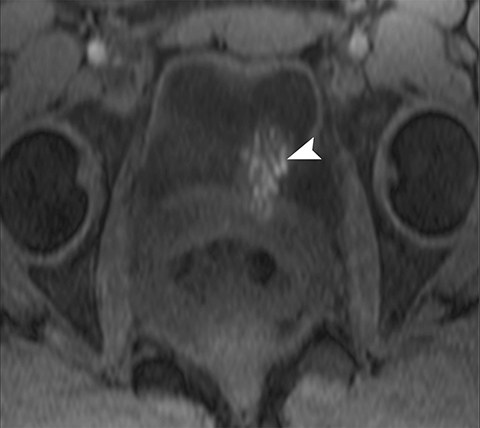
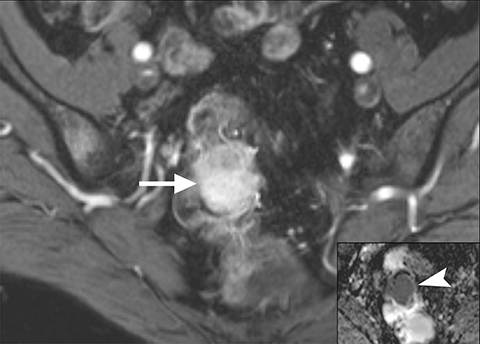
Common locations for DIE include the uterosacral ligaments, anterior rectosigmoid colon, bladder, rectovaginal septum, round ligaments, and muscular wall of pelvic organs. It can also be found in scar tissue, and has been reported in C-section scars within the uterine wall or anterior abdominal wall (Figure 4).11 Uterosacral ligaments are the most common location for DIE (Figure 5). With disease involvement, the ligaments can become thickened and develop adhesions to surrounding structures. MRI is noted to have a 69% sensitivity and >90% specificity for diagnosing uterosacral ligament deep infiltrating endometriosis, and is better than endorectal and endovaginal ultrasound.9 DIE can deeply invade into the muscularis propria of the rectosigmoid colon and this deep invasion typically requires surgical resection. At MRI, this has been described as a “mushroom cap” sign where the low intensity of the mushroom base is attributed to hypertrophy and fibrosis of the muscularis propria and the high signal intensity of the mushroom cap is attributed to the intact, overlying mucosa and submucosa which are displaced into the bowel lumen (Figure 6) .9 Bladder involvement with DIE usually involves the posterior wall and can result in partial or complete obliteration of the vesicouterine pouch. Similar to DIE in other locations, bladder involvement with DIE can appear as T2W hypointense infiltrative or nodular lesions centered in the vesicouterine pouch. There can be variable foci of T1W and T2W signal intensity within these DIE lesions, which represent the trapped endometrial glands (Figure 7). The round ligament is another site which can be involved with DIE, with one study of women undergoing laparoscopy for DIE showing involvement of the round ligament in 15% of cases.9 At MRI, these ligaments can become thickened and nodular with low T2W signal intensity and enhancement postcontrast similar to DIE lesions elsewhere in the pelvis.
Decidualized endometriosis
During pregnancy, hormonal changes cause proliferation of stromal cells within ectopic endometrial glands, known as decidualization. This proliferation of endometrial cells can cause appearance of solid mural nodules within endometriomas and can be mistaken for malignancy. Differentiation of mural nodules secondary to decidualized endometriosis versus malignancy is extremely challenging with imaging. Diffusion restriction and contrast enhancement within the mural nodules has been noted with both entities. There is suggestion that the T2W hyperintensity is slightly higher in mural nodules of decidualized endometriosis, with T2W hyperintense nodules being characteristic of decidual changes.12,13 The signal intensity of the nodules may also follow the signal of the decidualized endometrium (Figure 8).14 In such cases, close follow up is recommended to exclude malignancy. Decidualized endometriosis typically regresses or resolves after childbirth or termination of pregnancy.9
Secondary signs of endometriosis
Several findings are highly associated with endometriosis. While the below described conditions or observations may occur in isolation of endometriosis, if present, a thorough search pattern for endometriosis detection is recommended.
Hematosalpinx/hydrosalpinx
Approximately 30% of women with endometriosis demonstrate involvement of the fallopian tube at laparoscopy. Recurrent hemorrhage within implants located on the serosal surface of the fallopian tubes can lead to adhesions and tubal obstruction resulting in a dilated fallopian tube. On MRI, the tube may or may not demonstrate T1W hyperintensity suggestive of hemorrhagic contents. A study showed that in women with endometriosis and a dilated fallopian tube, only 40% of fallopian tubes demonstrated T1W hyperintensity, while 60% of fallopian tubes had imaging characteristics of a simple hydrosalpinx on imaging.9 It is also noted that T2 shading is often not seen in fallopian tubes associated with endometriosis as the endometrial implants may be located on the serosal surface of the fallopian tubes and not within the lumen of the tube. The main differential consideration in such cases is hydrosalpinx or pyosalpinx secondary to pelvic inflammatory disease.
Kissing ovaries
When endometriosis involves both ovaries and often also the posterior cul-de-sac, adhesions can pull the ovaries toward each other and toward the midline, an appearance called “kissing ovaries.”15 In this configuration, the ovaries are often posteriorly located and often abut the uterine serosa posteriorly (Figure 2).
Adenomyosis
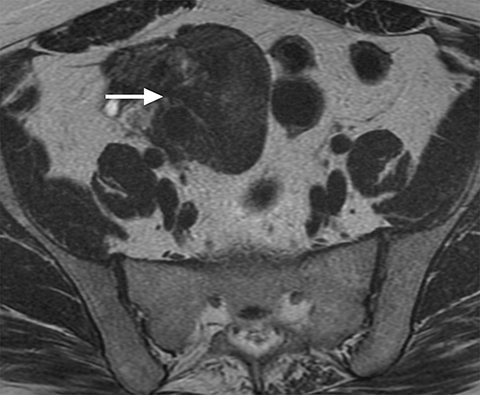
Adenomyosis is defined as the presence of heterotopic endometrial glands and stroma in the myometrium with adjacent smooth muscle hyperplasia.16 Many studies have illustrated an association of endometriosis and adenomyosis, the extent of which is variable in the literature, ranging from 10-80% in older studies.17 More recent studies analyzed the association between the two entities based on the thickness of the junctional zone which defines adenomyosis. In studies that use ≥12 mm of junctional zone thickness to define adenomyosis, one study reported a 34.6% association between the two entities while another reported a 58% association. It was also noted that the association can be as high as 79% if junctional zone thickness of ≥10 mm was used for the diagnosis of adenomyosis (Figure 4).17
Endometriosis-associated malignancy
Cancers arising in ovarian or extraovarian endometriosis is rare, seen in 1% of women with endometriosis. However, the prevalence of ovarian cancers among women with endometriosis is higher than in the general population with standardized incidence ratios of 1.4 – 4.2. These cancers have a different histologic profile and better prognosis than other more common epithelial ovarian cancers.18
Endometrioma-associated ovarian cancers are typically endometrioid (66.7%) or clear cell (14.8%) histology. On MRI, an enhancing mural nodule within an endometrioma is the most sensitive feature for endometrioma-associated cancer. It has a 97% sensitivity but only a 56% specificity as enhancing nodules can also be seen in benign entities such as inflammation, polypoid endometriosis, or decidual reaction of pregnancy.18 Because of intrinsic high T1W signal intensity of the cyst portion of the endometriomas, mural nodules are best seen on subtraction imaging and therefore obtaining pre- and postcontrast sequences is paramount in assessment of malignancy within endometriomas. Other imaging signs which may be less reliable include absence of T2 shading within an endometrioma, mural nodule diameter >3cm, and interval increase in size of the cyst. If present, secondary findings of malignancy include ascites and peritoneal implants.18 Endometriosis-associated cancer can arise in extraovarian sites in approximately 25% of cases, with sites of involvement and their reported frequency as follows: rectovaginal area (36%), colorectal region (11%), bladder (9%), vagina (7%), pelvic ligaments (4%), umbilicus (4%), cervix (4%), and fallopian tube (4%). They should be suspected when solid masses are seen in these locations on MRI with intermediate T1W and T2W signal intensity (Figure 9). They demonstrate contrast enhancement and diffusion restriction. Definitive diagnosis requires histologic sampling as diffusion restriction within these lesions is not definitive for malignancy and can be seen in benign endometriosis. Metastatic spread can occur via hematologic or lymphatic routes but can also occur by perineural extension.18
Conclusion
Endometriosis is a complex disease process with a heterogeneous phenotype that ranges from superficial implants to deep infiltrative disease of various organs of the pelvis and beyond. The imaging features may be highly varied, from classic ovarian endometriomas to DIE of the bowel, bladder, ureters, uterus, pelvic ligaments, abdominal wall and pelvic nerves. The radiographic appearance depends on the acuity or chronicity of repeat hemorrhage and on the degree of fibrosis. Although endometriosis is a benign disease, it rarely undergoes malignant degeneration. The role of MRI in the diagnosis and management of endometriosis is gaining recognition and becoming increasingly prevalent.
References
- Bennett GL, Slywotzky CM, Cantera M, et al. Unusual manifestations and complications of endometriosis–spectrum of imaging findings: pictorial review. AJR Am J Roent 2010;194(6 Suppl):WS34-46.
- Agarwal N, Subramanian A. Endometriosis - morphology, clinical presentations and molecular pathology. J Lab Physicians. 2010;2(1):1-9.
- Coutinho A, Jr., Bittencourt LK, Pires CE, et al. MR imaging in deep pelvic endometriosis: a pictorial essay. RadioGraphics. 2011;31(2):549-567.
- Hsu AL, Khachikyan I, Stratton P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin Obstet Gynecol. 2010;53(2):413-419.
- Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: diagnosis with MR imaging. Radiology. 1991;180(1):73-78.
- Theodoridis TD, Zepiridis L, Mikos T, et al. Comparison of diagnostic accuracy of transvaginal ultrasound with laparoscopy in the management of patients with adnexal masses. Arch Gynecol Obstet. 2009;280(5):767-773.
- Corwin MT, Gerscovich EO, Lamba R, et al. Differentiation of ovarian endometriomas from hemorrhagic cysts at MR imaging: utility of the T2 dark spot sign. Radiology. 2014;271(1):126-132.
- Outwater E, Schiebler ML, Owen RS, et al. Characterization of hemorrhagic adnexal lesions with MR imaging: blinded reader study. Radiology. 1993;186(2):489-494.
- Siegelman ES, Oliver ER. MR imaging of endometriosis: ten imaging pearls. RadioGraphics. 2012;32(6):1675-1691.
- Tanaka YO, Okada S, Yagi T, et al. MRI of endometriotic cysts in association with ovarian carcinoma. AJR Am J Roentgenol. 2010;194(2):355-361.
- Gougoutas CA, Siegelman ES, Hunt J, et al. Pelvic endometriosis: various manifestations and MR imaging findings. AJR Am J Roentgenol. 2000;175(2):353-358.
- Izza Rozalli F, Rahmat K, Fadzli F, et al. Decidualized ovarian endometrioma in a pregnant woman mimicking ovarian malignancy: magnetic resonance imaging and ultrasonographic findings. Iran J Radiol. 2015;12(4):e21260.
- Takeuchi M, Matsuzaki K, Uehara H, et al. Malignant transformation of pelvic endometriosis: MR imaging findings and pathologic correlation. RadioGraphics. 2006;26(2):407-417.
- Chaudhry S, Glanc P, Salem S. Detection and differential diagnosis of suspected malignant transformation of an endometrioma during pregnancy. BMJ Case Rep. 2013;2013:bcr2012007744.
- Dallaudiere B, Salut C, Hummel V, et al. MRI atlas of ectopic endometriosis. Diagn Interv Imaging. 2013;94(3):263-280.
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. RadioGraphics. 1999;19 (Suppl 1) Spec No:S147-160.
- Leyendecker G, Bilgicyildirim A, Inacker M, et al. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet. 2015;291(4):917-932.
- McDermott S, Oei TN, Iyer VR, et al. MR imaging of malignancies arising in endometriomas and extraovarian endometriosis. RadioGraphics. 2012;32(3):845-863.
Dr. Samreen, Dr. Bookwalter, Dr. Sheedy, and Dr. VanBuren are Radiologists, and Dr. Burnett is a Gynecologist, with the Mayo Clinic, Rochester, MN; Dr. Feldman is a Radiologist with the Cleveland Clinic, Cleveland, OH; and Dr. Menias is a Radiologist with the Mayo Clinic, Scottsdale, AZ.The authors have no conflicts of interest to disclose.
