Update: Radiologic-pathologic correlation of hepatocellular adenoma
By Dhingra S, Thupili C, Chua S, Shirlakar K, Prasad SR, Surabhi VR




Hepatocellular adenoma (HA) is a relatively uncommon benign liver neoplasm that is typically seen in obese women of childbearing age who are on long-term oral contraceptives.1 It is also reported to occur in men secondary to androgen drug use and in patients with rare metabolic disorders such as glycogen storage disease, maturity onset diabetes of the young and metabolic syndrome.2-4,5 Accurate identification and characterization of HA is clinically relevant as there is an increased tendency of this distinctive tumor to rupture and cause hemorrhage, to increase in size during pregnancy as well as to undergo malignant transformation.4 Recent patho-genetic studies have revealed that HA is a heterogeneous entity that may be classified into specific subtypes based on unique molecular signatures, histological features and immunohistochemistry.1,6-9 Based on characteristic MRI characteristics, specific HA subtypes may be identified non-invasively.10,11 Radiologists and pathologists have an equally important role to play in the diagnosis and morpho-molecular subtyping of HAs. This review focuses on the radiological and pathological features of HAs and its morphologic subtypes, and in addition dwells on the differential diagnosis of HAs based on imaging characteristics.
Epidemiology and etiologic associations
Hepatocellular adenoma has an incidence of 1–1.3 million cases per year in North America and Europe.12 Several studies have consistently linked the occurrence of HAs to oral contraceptive pill (OCP) use.13-15 The risk of HA is associated with dose and duration of oral contraceptive use; HAs were particularly associated with use of older generation of OC pills with high-estrogen content. Regression of Hepatocellular adenoma has been reported to occur following cessation of OCPs.16 HAs have also been reported to occur in men secondary to anabolic steroid/androgen use.2,3 Other hormonal therapies and risk factors include: clomiphene, danazol,5 testosterone in patients with Fanconi anemia (FA) and without FA, Klinefelter’s syndrome, Glycogen storage disorders I, III and IV, alcohol, and metabolic syndrome. Hepatocellular adenomatosis, defined as development of >10 HAs in a patient, is usually related to germline mutations of HNF1-α gene and is also seen in patients with type 3 maturity onset diabetes of young (MODY 3).17
Clinical presentation
The majority of HAs are clinically occult and are incidentally detected on imaging. The risk factors for hemorrhage are size >5 cm, a subcapsular location and longstanding OCP use.10,18,19 HCA associated with hemorrhage may present with acute abdominal pain, elevated liver enzymes, and hypovolemic shock. Inflammatory HCA has the highest risk of hemorrhage and may show elevated levels of acute-phase reactants. The β-catenin activated subtype has the highest predilection for malignant transformation of all HCAs and this may be seen as a rapid increase in the size of a previously known HCA.10,18,19
Classification
Hepatocellular adenoma (HA) was considered a homogeneous entity until the early 2000s, when a French group of clinical researchers demonstrated somatic bi-allelic mutations of transcription factor 1 (TCF 1) gene encoding hepatocyte nuclear factor 1 (HNF 1) in a subset of HAs.20 Subsequently, they reported that telangiectatic focal nodular hyperplasias (T-FNHs) are monoclonal lesions and clinically behave similar to HAs without HNF-1-α mutation.21 These were later classified as inflammatory HAs.22 Ongoing genomic studies of HAs by the same French group led to the landmark paper in 2006 that introduced the genotype-phenotype taxonomy of HAs.22According to this schemata, the HAs are classified into 4 subtypes, of which the first three have unique molecular signatures as well as histological, immunophenotypical and radiological features. 7 These were: 1. HNF-1-α (HNF 1A) mutated HAs; 2. β-catenin mutated HAs; 3. Inflammatory-type HAs. The last group classified as “unclassified” refers to HAs that do not satisfy the diagnostic criterion described in the three other subtypes (Table 1). Using sequencing and gene expression profiling, this classification has further been refined recently to include 6 subgroups of HAs.9, 1, 23 The β-catenin mutated HAs (BHA and IHA with β-catenin mutations) are further split into: (a) BHA with exon 3 mutations; (b) BHA with exon 7/8 mutations. A subset of unclassified HA has been found to show constitutive activation of sonic hedgehog (shh) pathway due to overexpression of GLI family zinc finger 1 (GLI1). These HAs are associated with a higher risk of bleeding. They do not have specific morphological features or immunoexpression profiles.
We will focus on four subtypes of HAs in this review, as radiologic features of the newer subtypes are not well defined. Clinical implementation of this classification in diagnostic medicine has brought about a paradigm shift in the management of HAs, as this helps determine the potential for complications including bleeding and malignant transformation, and thereby guide further management in terms of resection and/or surveillance.
Pathology
Gross evaluation
Hepatocellular adenomas are well-demarcated lesions that can occasionally be encapsulated and range in size from 1-30 cm.8 HA may present as a solitary mass or as multifocal, variably sized, soft tissue masses. They typically arise in non-fibrotic liver, however, they may occur in the background of cirrhosis. HAs reported in the setting of cirrhosis are usually of the inflammatory subtype.24-26 Rare cases of multiple HNF-1-α mutated HAs has been reported to occur in the background of congenital hepatocellular fibrosis.27 The cut surface of HA may be tan-yellow or red-brown depending upon the presence of steatosis or peliosis/hemorrhage/old hemorrhage, respectively. Inflammatory HA shows alternating pale red and dark-red surface.8
Microscopic evaluation
Hepatocellular adenoma is classically characterized by sheets of benign-appearing hepatocytes with interspersed thin-walled, unpaired arteries. The hepatocyte trabeculae are 1-3 cells thick and the reticulin framework is preserved. Portal tracts containing portal veins or bile ducts are absent. Few randomly distributed pseudo-portal tract areas with thick walled vessels and ductular reaction can be seen in some HAs. Other variable features include steatosis, inflammatory cell infiltrate, sinusoidal dilatation, myxoid changes(28) and presence of pigments such as bile pigment, lipofuscin or Dubin Johnson-like pigment.29,30
Radiologic and pathologic features of subtypes of hepatocellular adenoma
Hepatocyte nuclear factor-1-α (HNF1A) mutated Hepatocellular adenoma
These comprise 35% to 40% of all HA. Their molecular signature is characterized by bi-allelic inactivating mutations of TCF-1, a tumor suppressor gene, located on long arm of chromosome 12, and encodes for transcription factor HNF-1A, which is involved in hepatocyte differentiation and expression of certain genes encoding for albumin, β-fibrinogen and α-1-antirypsin.20 The mutations are largely somatic, however, germline mutations of TCF1 (HNF1A) gene are associated with type 3 maturity onset diabetes of the young (MODY 3). This is an autosomal dominant disease, which presents in early adulthood and is associated with development of familial adenomatosis.17 HNF1A mutation down-regulates liver type-fatty acid binding protein (L-FABP) and causes lipogenesis by promoting fatty acid synthetase.31
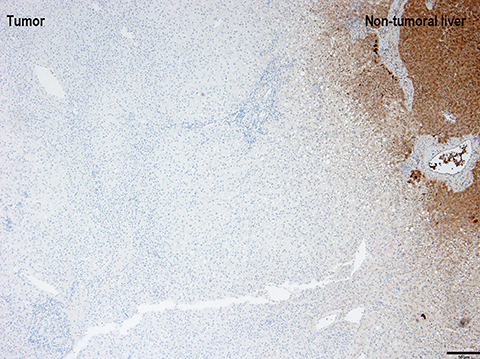
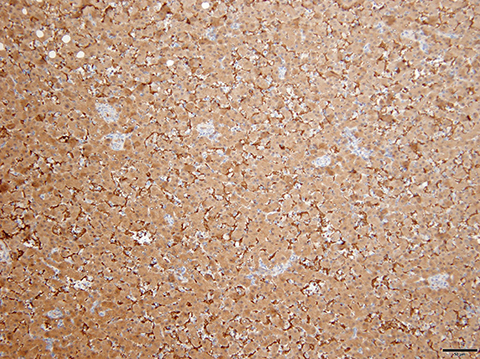
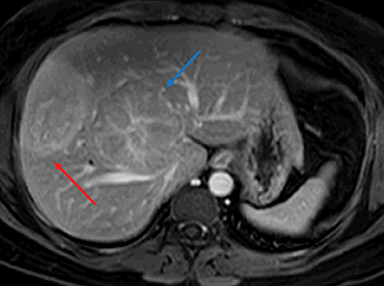
Gross examination of HNF1A mutated HA shows a well-demarcated solid tumor with a tan-yellow soft to firm cut surface. The phenotypic correlate of above-mentioned molecular alterations is presence of moderate to diffuse steatosis in majority of HNF1A mutated HA. No significant inflammation or cellular atypia is seen. Immunostaining with antibodies to L-FABP shows loss of expression in the HA, whereas, the expression is preserved in the surrounding non-neoplastic liver parenchyma (Figure 1).
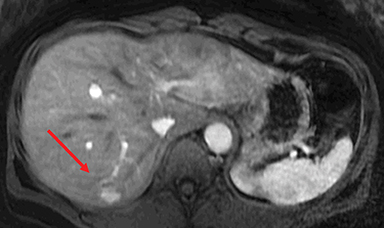
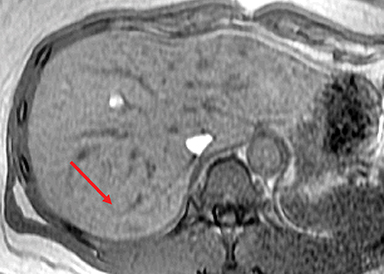
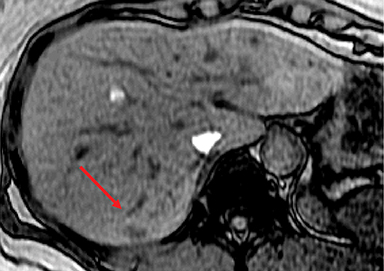
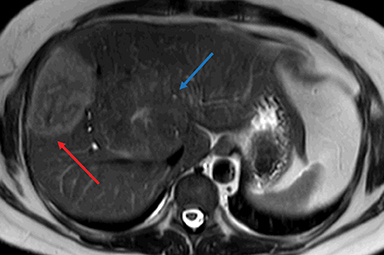
HNF-1α–mutated HAs are associated with MODY type 3 and hepatic steatosis. MR imaging findings include isointense signal on T2WI, isointense on T1WI and typically show intracellular lipid as evidenced by diffuse signal drop-off on a chemical shift sequence (Figure 2).10,18,19,32 HNF-1α–mutated HAs demonstrate intense enhancement in the arterial phase and become isointense to the liver parenchyma in the portal venous and delayed phases. These lesions remain hypointense on HBP phase with hepatocyte specific gadolinium agents. Tumors less than 5 cm in maximum dimension show minimal risk of bleeding and carry minimal or no risk for malignancy.10,18,19
Inflammatory hepatocellular adenoma (IHCA)
Historically, IHCAs were referred to as telangiectatic FNHs until 2004-2005, when studies showed these to be monoclonal neoplasms, which behave biologically similar to HAs. IHCA comprise about 30-35% of all HAs. This subtype of HA is characterized by activation of signal transducer and activation of transcription 3 (STAT 3) signaling pathway leading to induction of acute phase inflammatory response within the tumoral hepatocytes. About two-thirds of IHCA show mutations of interleukin 6-signal transducer gene (IL6ST gene), which encodes for glycoprotein 130 (gp130), a component of IL-6 receptor. Activation of IL-6 promotes the STAT3 signaling pathway. About one-third of the HAs do not show mutations of IL6ST gene, yet, show evidence of STAT 3 activation and gp130 protein expression through unknown mechanisms. About 10% of IHCA show concomitant β-catenin mutations.
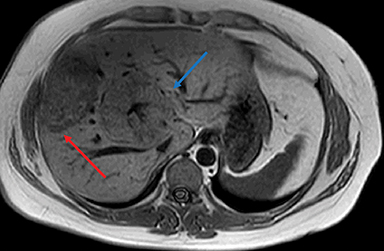
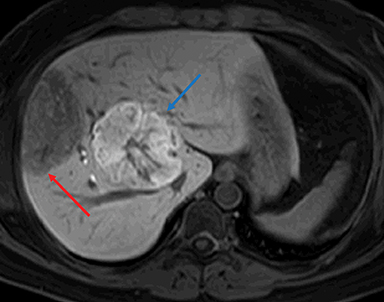
Gross characteristics of IHCA include a well-demarcated tumor with red-brown variegated cut surface. Histologically, these are characterized by sinusoidal dilatation, peliosis, presence of pseudo-portal tracts like areas with dystrophic blood vessels, ductular reaction and varying degree of inflammatory infiltrates. Focal steatosis may be seen in some cases. Immunohistochemical staining with antibodies to acute phase inflammatory reactants, serum amyloid A (SAA) and C reactive protein (CRP), show diffuse cytoplasmic expression of both SAA and CRP. Immunostain for L-FABP shows retained cytoplasmic expression in tumoral hepatocytes (Figure 3). IHCA with β-catenin mutations show nuclear expression of β-catenin on immunohistochemical staining. Glutamine synthetase is a surrogate marker of β-catenin mutation.33 Some IHCAs with β-catenin mutations maybe positive or negative for nuclear β-catenin expression on immunostaining, but will show strong diffuse/patchy cytoplasmic staining for glutamine synthetase.34 These β-catenin mutations may occur on exon 3 or exon 7/8. 1,23
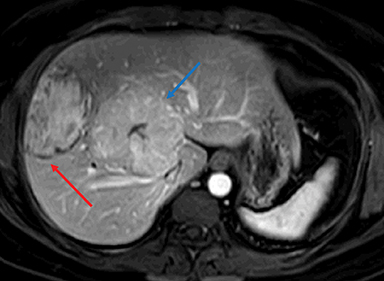
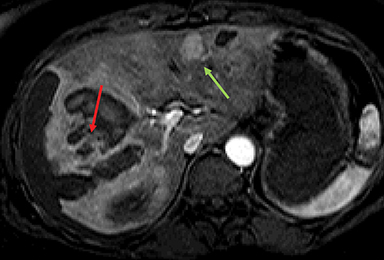
Inflammatory HAs show mild to moderate hyperintense signal on T2WI and are iso-intense or mildly hyperintense on T1WI with no significant signal drop-off with chemical shift imaging. 32 After administration of extracellular gadolinium-based contrast material, IHAs usually show intense enhancement during the arterial phase, with persistent enhancement in the portal venous and delayed phases. IHAs may show uptake of hepatobiliary specific gadolinium agent and may show peripheral rim like uptake or heterogeneous internal retention of contrast on the hepatobiliary phase. 32,35 (Figure 4). Inflammatory HAs show the highest risk of bleeding due to the presence of sinusoidal dilatation, which can occur in about 30% of these tumors and particularly seen in tumors larger than 5 cm maximum dimension and subcapsular tumors (Figure 5). About 10% of inflammatory HAs show an increased risk of malignancy. 10,18,19
β-catenin activated hepatocellular adenoma (BHA)
These adenomas comprise about 20% of all HAs. 8 Wnt/β-catenin pathway is involved in hepatocellular development and zonation. In normal, non-neoplastic hepatocytes, activation of β-catenin protein is transient followed by degradation. Mutations of β-catenin gene (CTNNB1 gene) lead to production of mutant protein that has prolonged half-life and is resistant to degradation.36 In 10% of cases, there are deletions CTNNB1 exon 3 leading to decrease in β-catenin degradation.1,23 In other cases, there are point mutations targeting hotspots in exon 3 or those in exon 7/8.4,9
Gross characteristics of β-catenin mutated HA are unremarkable. They may present as well-demarcated tumors with fleshy cut surface. Histologically, they lack a distinctive morphology. They are composed of sheets of hepatocytes with interspersed unpaired arteries. Some may show cytologic and architectural atypia characterized by pseudoacinar formation. Steatosis is not a typical feature. Immunostain for beta-catenin will show nuclear beta-catenin staining, however, this can be very focal in distribution. Glutamine synthetase is a surrogate marker for β-catenin mutation. Immunostain for glutamine synthetase shows strong diffuse cytoplasmic staining, however, the staining can be heterogeneous and variable.23
No specific imaging findings have been reported to diagnose β-catenin–mutated hepatocellular adenomas on imaging. T1 and T2 signal of these tumors is variable depending on the presence of hemorrhage and/or necrosis.10,18,19 β-catenin–mutated HAs commonly demonstrate strong arterial enhancement with portal venous washout and no uptake on hepatobiliary phase (Figure 6). The risk of hepatocellular carcinoma is about 5%–10% in these HAs. β-catenin–mutated HAs carry the highest risk of malignancy.10,18,19
Hepatocellular adenoma unclassified
Unclassified HA constitutes 10% of all HAs and these tumors do not show any specific genetic abnormalities. No specific MR imaging findings are reported to identify unclassified HAs.10,18,19
Conclusion
Hepatocellular adenomas are a diverse group of benign liver neoplasms with increased propensity to bleed and to undergo malignant transformation. Recent genetic studies have identified several subtypes with distinctive tumor genetics and pathways as well as specific risk factors and risks of complications. MRI plays a key role in the diagnosis and classification of HAs as well as in surveillance. Hepatocellular adenomas with β-catenin mutations frequently undergo malignant change, inflammatory HAs commonly bleed, and steatotic HAs typically portend a favorable prognosis. A knowledge of the clinical and imaging findings and associated complications of the subtypes of hepatocellular adenoma permit optimal patient management.
References
- Bioulac-Sage P, Sempoux C, Balabaud C. Hepatocellular adenoma: Classification, variants and clinical relevance. Semin Diagn Pathol. 2017;34(2):112-125.
- Velazquez I, Alter BP. Androgens and liver tumors: Fanconi’s anemia and non-Fanconi’s conditions. Am J Hematol. 2004;77(3):257-267.
- Gupta S, Naini BV, Munoz R, et al. Hepatocellular neoplasms arising in association with androgen use. Am J Surg Pathol. 2016;40(4):454-461.
- Nault JC, Couchy G, Balabaud C, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152(4):880-894.
- Kahn H, Manzarbeitia C, Theise N, et al. Danazol-induced hepatocellular adenomas. A case report and review of the literature. Arch Pathol Lab Med. 1991;115(10):1054-1057.
- Bioulac-Sage P, Cubel G, Balabaud C, et al. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis. 2011;31(1):91-103.
- Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46(3):740-748.
- Dhingra S, Fiel MI. Update on the new classification of hepatocellular adenomas: Clinical, molecular, and pathologic characteristics. Arch Pathol Lab Med. 2014;138(8):1090-1097.
- Nault JC, Paradis V, Cherqui D, et al. Molecular classification of hepatocellular adenoma in clinical practice. J Hepatol. 2017;67(5):1074-1083.
- Shanbhogue AK, Prasad SR, Takahashi N, et al. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology. 2011;258(3):673-693.
- Ba-Ssalamah A, Antunes C, Feier D, et al. Morphologic and molecular features of hepatocellular adenoma with gadoxetic acid-enhanced MR imaging. Radiology. 2015;277(1):104-113.
- Dharmana H, Saravana-Bawan S, Girgis S, et al. Hepatocellular adenoma: imaging review of the various molecular subtypes. Clin Radiol. 2017;72(4):276-285.
- Model DG, Fox JA, Jones RW. Letter: Multiple hepatocellular adenomas associated with an oral contraceptive. Lancet. 1975;305(7911):865.
- Ameriks JA, Thompson NW, Frey CF, et al. Hepatocellular cell adenomas, spontaneous liver rupture, and oral contraceptives. Arch Surg. 1975;110(5):548-557.
- Christopherson WM, Mays ET, Barrows GH. Liver tumors in women on contraceptive steroids. Obstet Gynecol. 1975;46(2):221-223.
- Sinclair M, Schelleman A, Sandhu D, et al. Regression of hepatocellular adenomas and systemic inflammatory syndrome after cessation of estrogen therapy. Hepatology. 2017;66(3):989-991.
- Bacq Y, Jacquemin E, Balabaud C, et al. Familial liver adenomatosis associated with hepatocyte nuclear factor 1 alpha inactivation. Gastroenterology. 2003;125(5):1470-1475.
- Katabathina VS, Menias CO, Shanbhogue AK, et al. Genetics and imaging of hepatocellular adenomas: 2011 update. Radiographics. 2011;31(6):1529-1543.
- Shanbhogue A, Shah SN, Zaheer A, et al. Hepatocellular adenomas: current update on genetics, taxonomy, and management. J Comput Assist Tomogr. 2011;35(2):159-166.
- Bluteau O, Jeannot E, Bioulac-Sage P, J et al. Bi-allelic inactivation of TCF1 in hepatocellular adenomas. Nat Genet. 2002;32(2):312-315.
- Bioulac-Sage P, Rebouissou S, Sa Cunha A, et al. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology. 2005;128(5):1211-1218.
- Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology. 2006;43(3):515-524.
- Bioulac-Sage P, Sempoux C, Balabaud C. Hepatocellular adenomas: Morphology and genomics. Gastroenterol Clin North Am. 2017;46(2):253-272.
- Sasaki M, Nakanuma Y. Serum amyloid A-positive hepatocellular neoplasm: A new type of tumor arising in patients with advanced alcoholic disease. Dig Dis. 2015;33(5):648-654.
- Calderaro J, Nault JC, Balabaud C, et al. Inflammatory hepatocellular adenomas developed in the setting of chronic liver disease and cirrhosis. Mod Pathol. 2016;29(1):43-50.
- Gordic S, Thung SN, Roayaie S. Hepatocellular adenomatosis in liver cirrhosis. Eur J Radiol Open. 2017;4:115-117.
- Paradis V, Bioulac-Sage P, Balabaud C. Congenital hepatocellular fibrosis with multiple HNF1alpha hepatocellular adenomas. Clin Res Hepatol Gastroenterol. 2014;38(6):e115-116.
- Salaria SN, Graham RP, Aishima S. Primary hepatocellular tumors with myxoid change: morphologically unique hepatocellular adenomas and hepatocellular carcinomas. Am J Surg Pathol. 2015;39(3):318-324.
- Hechtman JF, Raoufi M, Fiel MI, et al. Hepatocellular carcinoma arising in a pigmented telangiectatic adenoma with nuclear beta-catenin and glutamine synthetase positivity: Case report and review of the literature. Am J Surg Pathol. 2011;35(6):927-932.
- Coelho R, Goncalves R, Carneiro F, et al. Pigmented hepatocellular adenoma with beta-catenin activation: Case report and literature review. Ann Hepatol. 2016;15(4):598-603.
- Rebouissou S, Imbeaud S, Balabaud C, et al. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem. 2007;282(19):14437-14446.
- Glockner JF, Lee CU, Mounajjed T. Inflammatory hepatocellular adenomas: Characterization with hepatobiliary MRI contrast agents. Magn Reson Imaging. 2017;47:103-110.
- Balabaud C, Bioulac-Sage P. Glutamine synthetase interpretation in hepatocellular adenoma. Virchows Arch. 2014;465(4):495-496.
- Berry RS, Gullapalli RR, Wu J. Diffuse glutamine synthetase overexpression restricted to areas of peliosis in a beta-catenin-activated hepatocellular adenoma: a potential pitfall in glutamine synthetase interpretation. Virchows Arch. 2014;465(2):241-245.
- Agarwal S, Fuentes-Orrego JM, Arnason T, et al. Inflammatory hepatocellular adenomas can mimic focal nodular hyperplasia on gadoxetic acid-enhanced MRI. AJR Am J Roentgenol. 2014;203(4):W408-414.
- Rogacki K, Kasprzak A, Stepinski A. Alterations of Wnt/beta-catenin signaling pathway in hepatocellular carcinomas associated with hepatitis C virus. Pol J Pathol. 2015;66(1):9-21.
Dr. Dhingra is an Associate Professor of Pathology at Baylor College of Medicine, Houston, TX; Dr. Surabhi is a Professor of Radiology; Dr. Thupili, Dr. Shiralkar and Dr. Chua are Assistant Professors of Radiology, in the Section of Abdominal Imaging, University of Texas Health Sciences Center at Houston, Houston, TX; and Dr. Prasad is a Professor of Radiology at The University of Texas MD Anderson Cancer Center, Houston, TX. The authors have no conflicts of interest to disclose.
