Imaging brachial plexus pathology
By Hallinan JTPD, Pathria MN, Huang BK
Brachial plexus disorders can be diagnostic challenges, owing to the region’s complex anatomy and nonspecific symptomatology. MRI remains the best modality for assessing the brachial plexus (BP), due to its superior soft-tissue contrast compared to CT or ultrasound. Trauma is the most common cause of BP dysfunction, closely followed by tumor infiltration. Infection, inflammation and iatrogenic causes are less common. This review article will provide an overview of anatomy and practical, up-to-date BP imaging techniques for general radiologists, followed by a step-wise discussion of common pathology. Clinically relevant advances such as dynamic thoracic outlet MRI will also be discussed.
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Anatomy
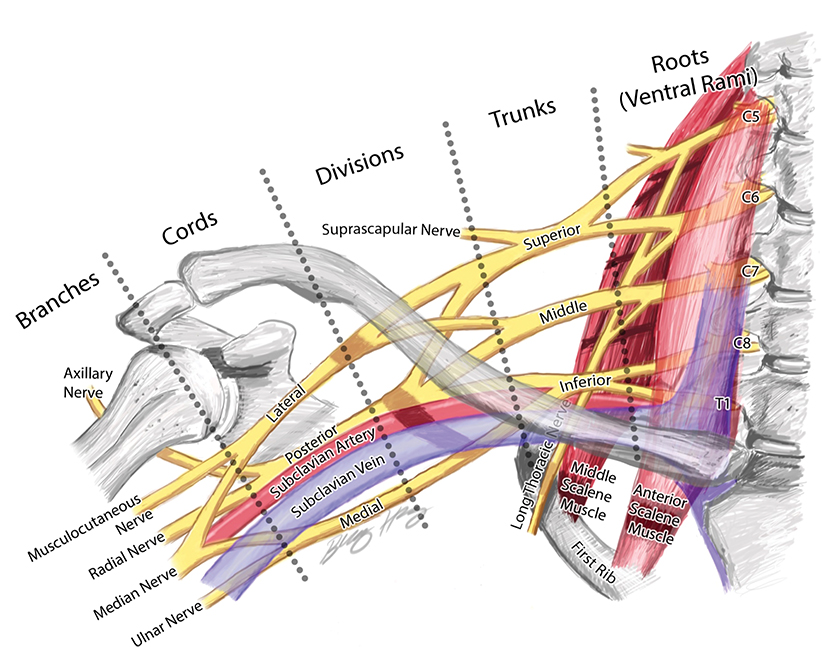
The BP provides motor innervation to the ipsilateral chest, shoulder, and upper limb with the exception of the trapezius, which is supplied by the spinal accessory nerve. It is divided anatomically into roots, trunks, divisions, cords, terminal and collateral branches (Figure 1). These constituents may best be recalled using this mnemonic: Radiology (roots) Technicians (trunks) Drink (divisions) Cold (cords) Brew (terminal branches) Coffee (collateral branches) —a slight divergence from the usual alcohol-based memory tools.
The BP roots arise from the C5-T1 ventral rami of the spinal cord, with variable C4 and T1 contributions. As a result, the “roots” are actually the ventral rami of the spinal nerves formed from the dorsal and ventral rootlets arising from the spinal cord.1 Although “roots” is a misnomer, the term is well established in the literature and will be used throughout this article.
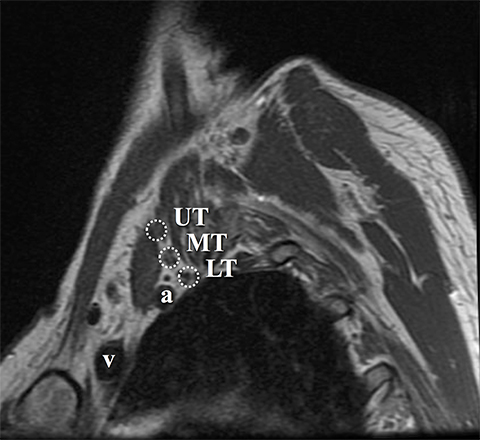
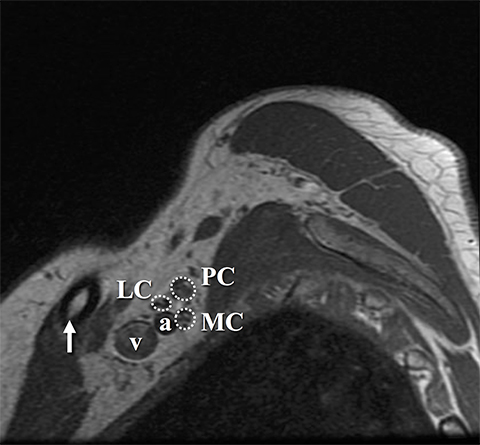
The roots exit through their respective neural foramina, and travel between the anterior and middle/posterior scalene muscles in the interscalene triangle, or space (Figures 2, 3). Lateral to the middle scalene muscle the three trunks are formed: C5 and C6 combine into the upper trunk, C7 continues alone as the middle trunk, and C8 and T1 combine to form the lower trunk (Figures 2, 4). The trunks traverse posterosuperior to the subclavian artery behind the clavicle and each divides into anterior and posterior divisions within the costoclavicular space. The vessels lie inferiorly at the costoclavicular space with the subclavian vein anterior to the artery. 2,3 This space, bounded by the clavicle superiorly, subclavius muscle anteriorly, and the first rib and middle scalene muscle posteriorly, is a common site of BP and subclavian vascular compression.
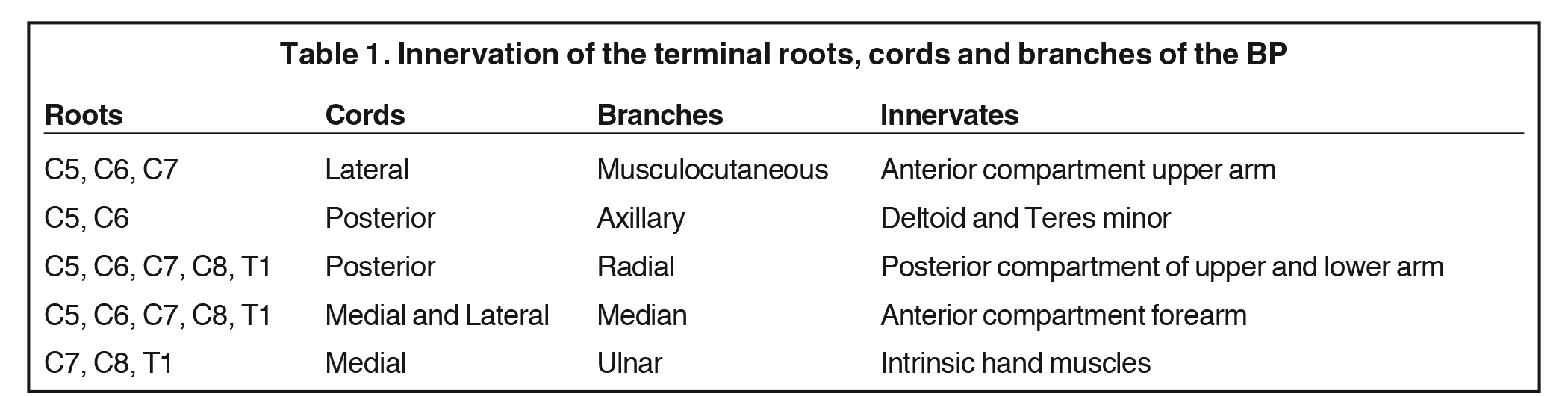
The conversion of the six divisions into three cords occurs at the lateral border of the first rib (Figure 5). The cords extend into the retropectoralis minor space and can be readily identified due to their relationship to the axillary artery; The medial cord lies posteroinferiorly, lateral cord anterosuperiorly and posterior cord posterosuperiorly. The cords divide into five major terminal branches at the lateral border of the pectoralis minor: the median, ulnar, radial, musculocutaneous and axillary nerves.4,5 In addition there are numerous (~11) collateral branches that exit along the BP more proximally, eg,, suprascapular nerve from the superior trunk. Table 1 shows the innervation of the cords and terminal branches.
MR imaging protocol
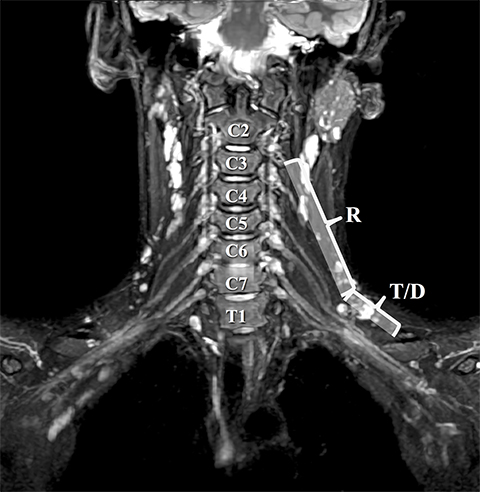
Accurate visualization of the BP from the roots to terminal branches must take into consideration its oblique superomedial to inferolateral course. As such, oblique coronal and sagittal images are preferred, but not mandatory. Our protocol utilizes initial large field of view images for bilateral comparison of the brachial plexuses. These are typically performed with T1W fast spin echo images and three-dimensional T2W fat-suppressed sequences, which allows for multiplanar reconstructions. In a high-volume facility, isotropic imaging can shorten imaging time and decrease the need for custom-tailored exams.4,6
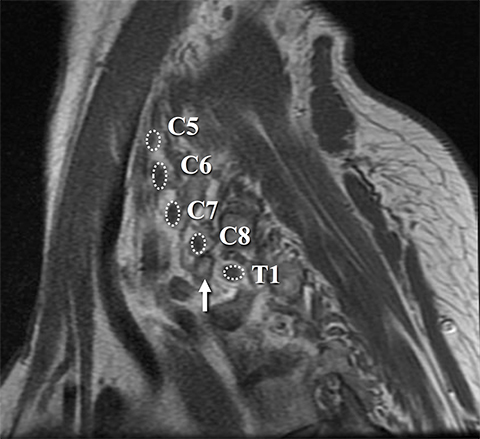
Dedicated, multiplanar, small field of view images of the affected plexus are then performed. Coronal T2W fat-saturated sequences are useful for assessment of abnormal neural edema and enlargement; T1W sagittal images are especially useful for assessment of fat planes along with surrounding bony and muscular anomalies; eg, cervical ribs. 7 High resolution, axial, pre-ganglionic, T2W images of the cervical region and nerve roots are also performed. These are useful for assessment of traumatic nerve root avulsions and pseudomeningoceles.3,5
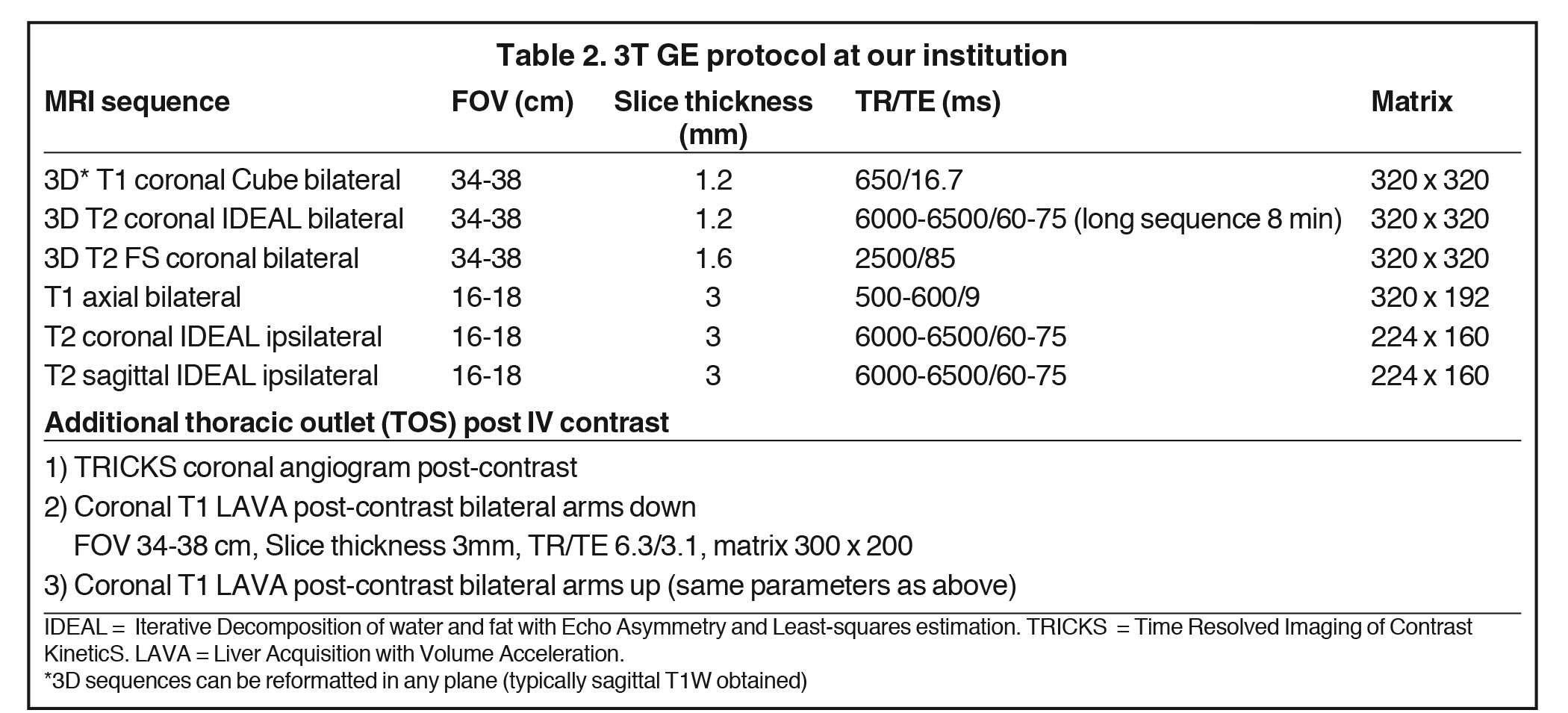
Both 1.5T and 3T platforms may be used, with a sample protocol shown in Table 2 (For 3T only). 3T is preferred due to better resolution and signal-to-noise profile over 1.5T, with 1.5T reserved for patients with hardware or contraindications to 3T. Neurovascular surface coils or a wrap-around body coil can be utilized.
Intravenous gadolinium-based contrast is not routinely required for BP imaging as most nerve pathology is well assessed on the high-resolution, fat-suppressed T2W images. Contrast administration is typically required in cases of suspected infection or tumor infiltration. Contrast allows for more accurate assessment of disease extent, identification of drainable collections and characterization of focal masses, such as neurogenic tumors.1,4
Another common indication for imaging is thoracic outlet syndrome (TOS). The aim is to assess for the site of vascular and/or neural compression, which is typically at the costoclavicular space and may involve anomalous ribs or musculature, or fibrous bands. 4,8 Provocative maneuvers, such as arm raising and head turning, can unmask the extent and location of the compression. Bilateral coronal T1W sequences and post-contrast MR angiography are commonly performed in each position (Table 2).
Diffusion tensor imaging (DTI) with fiber tractography has the potential to detect microstructural abnormalities beyond the resolution of conventional, anatomic MRI sequences. It is still an experimental tool, but has been shown to detect tract disruption, infiltration and internal disorganization, which could allow for earlier diagnosis of neoplastic or inflammatory neuropathies.5,9,10
CT of the brachial plexus
CT also plays a role in the diagnosis of BP pathology. Extension of neoplastic disease into the plexus (eg, Pancoast tumor), rib and clavicle anomalies, and adjacent fractures and hematomas can be visualized. CT angiography also provides a high-resolution and rapid assessment of arterial and venous patency in trauma and TOS.
Ultrasound of the brachial plexus
Ultrasound of the BP is technically feasible, but challenging due to the complex anatomy and bony relations.11,12 In skilled hands, it is a useful bedside tool for assessing traumatic BP injury and is routinely used by anesthesiologists to perform nerve blocks.13 It is also commonly used for dynamic vascular assessment in suspected TOS.14,15 Lapeque et al (2014) provide a practical review on BP ultrasound. However, for the majority of BP injuries, MRI remains the standard examination, with ultrasound as a useful adjunct.
Brachial plexus lesions
BP lesions can be classified broadly into two categories: traumatic and non-traumatic lesions.16 Traumatic lesions form the majority of BP injuries, and are the major indication for imaging. Non-traumatic lesions are a heterogeneous group encompassing neoplastic processes, infection/inflammation and functional neurovascular compression or TOS.17
Trauma
Motor vehicle accidents are responsible for the majority of traumatic BP injuries. Traumatic traction on the BP can lead to proximal spinal nerve root avulsion (pre-ganglionic) or more distal rupture (post-ganglionic).18
Clinical examination of the neurological injury should be documented promptly to determine the site and clinical course of lesions (recovery or stable deficit) and whether surgery is indicated. Upper limb paralysis is complete in approximately 75% of patients, with a supraclavicular site of injury seen in 72% of cases. 19 Delineation of the site of injury is important for prognosis and management. Pre-ganglionic injuries may require early (<3 months) reconstruction using nerve transfers, whereas postganglionic injuries may be repaired after a longer period of observation.1,5
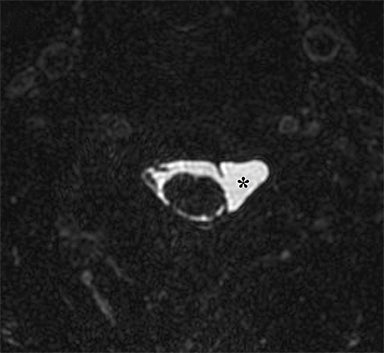
Identification of pre-ganglionic lesions requires high-resolution 3D T2W images, which are typically viewed in the axial plane (Figure 6). Intradural nerve rootlets should be assessed for integrity from the root entry zone to the dorsal root ganglion within the neural foramen. These injuries are associated with traumatic pseudomeningoceles in 80% of cases.1,5,12 Other common associated findings in pre-ganglionic avulsion include contralateral cord displacement, syrinx, epidural hematoma and spinal cord edema. The posterior paraspinal musculature should also be assessed for denervation edema, which can occur due to a nerve root avulsion at or before the dorsal root ganglion. Stretching or incomplete avulsion of the pre-ganglionic rootlets is more challenging to diagnose, and may be suggested when there is differential enhancement of the rootlets.1,5
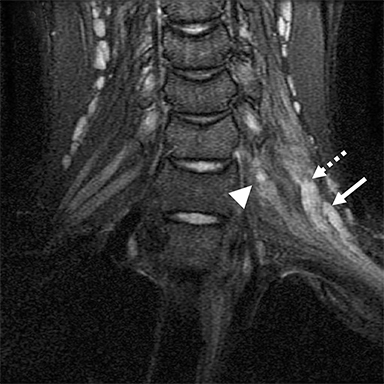
Post-ganglionic avulsions distal to the neural foramina typically occur proximally at the scalene triangle, but may variably involve the trunks to distal branches. These can also be partial or complete; complete ruptures are usually associated with distal nerve retraction (Figure 7). Another finding is nodular thickening and mild enhancement due to a post-traumatic neuroma in continuity. Indirect findings include formation of hematomas at the site of avulsion and denervation edema and enhancement at the shoulder girdle musculature.1,5,20
MRI is the mainstay for diagnosis, although ultrasound can provide an accessible bedside examination in critically injured patients. 11 Ultrasound can be used to assess for post-ganglionic injuries at the interscalene triangle or distal to the costoclavicular space. Thickening or discontinuity of the trunks or cords can be assessed using the contralateral side as a readily accessible control. 10,11,12 CT myelography also remains an accurate technique for assessing pre-ganglionic avulsions with clear depiction of the intradural rootlets.17,21
Plexus traction injuries in neonates are well-recognized, although they are uncommonly imaged. Traction on the superior plexus at C5-7 (Erb-Duchenne palsy) is more common than the inferior plexus at C8 and T1 (Klumpke palsy). These injuries are usually managed conservatively, with surgery reserved for those with poor functional recovery and suspected pre-ganglionic injury on imaging. 1,22
Malignant neoplasms
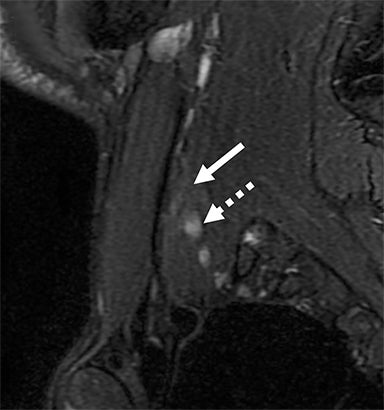
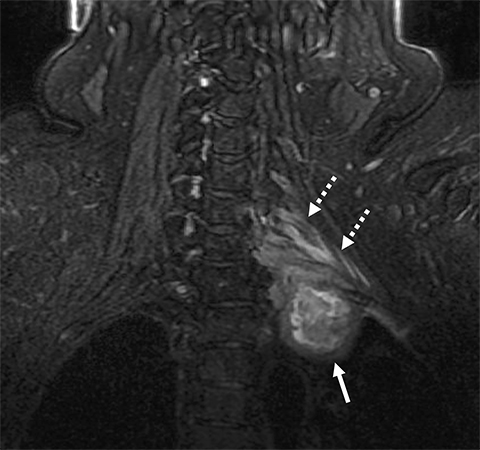
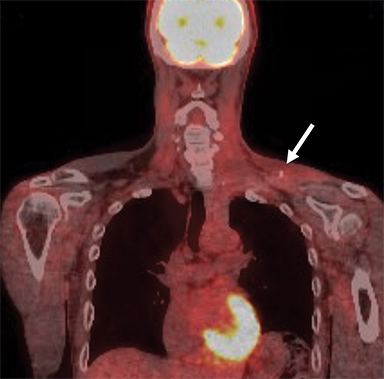
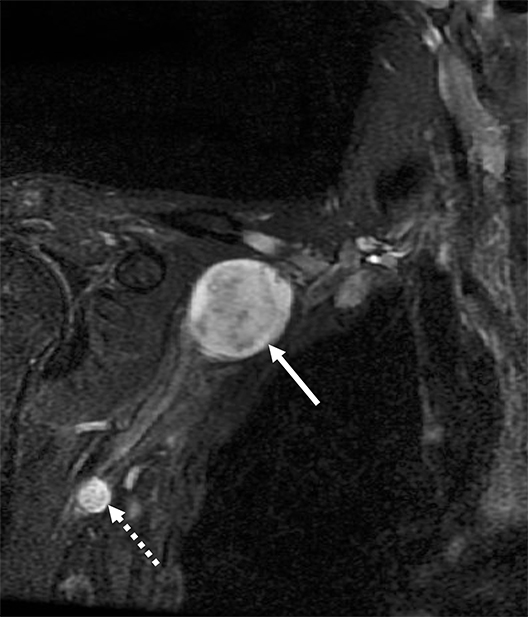
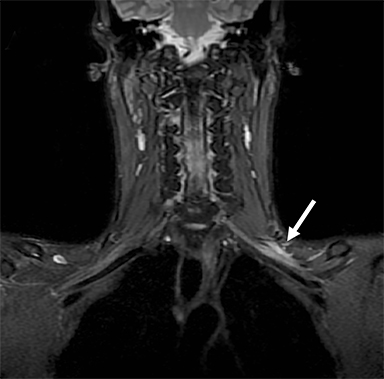
Malignant involvement of the BP is more common than primary benign lesions, and occurs in an older age group. The BP can be infiltrated by primary malignant tumors, local secondary malignancy or metastatic disease (Figure 8). Lesions arising directly from the plexus include malignant peripheral nerve sheath tumors and a plethora of uncommon sarcomatous lesions (eg, fibrosarcoma or radiation induced tumors). Local malignant extension commonly occurs secondary to a Pancoast tumor at the lung apex or due to a head and neck carcinoma (Figure 9). 1,5,15
These lesions can involve multiple structures including the vertebral bodies, ribs and clavicle with compression and direct invasion of the neural and vascular structures. Clinically, there is usually pain followed by paraesthesia and weakness at the upper limb, predominantly involving the inferior trunk and spinal nerves in a Pancoast tumor. In addition, adjacent infiltration of the sympathetic chain (stellate ganglion) may result in Horner’s syndrome.6,23
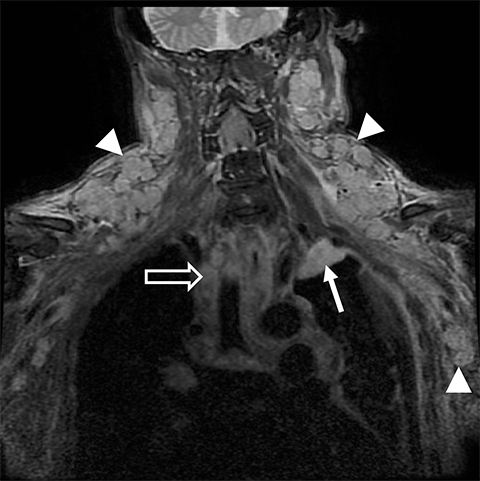
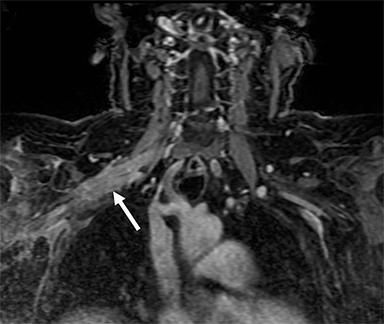
Metastatic disease of the BP commonly arises in the adjacent regional lymph nodes. In particular, breast carcinoma can metastasize along the axillary and supraclavicular nodes resulting in focal masses or even diffuse plexus infiltration (Figure 10). Head and neck carcinomas and lymphoma are other common causes of metastatic infiltration.16,17
MR imaging for suspected malignant involvement should include post-contrast sequences to assess for focal masses and any perineural or leptomeningeal enhancement extending to the spinal cord. The imaging report should also document potential involvement of the ipsilateral vertebral artery, which could be further evaluated using MR angiography.1,5,6
Radiation plexopathy
Any discussion of malignant infiltration at the BP is incomplete without considering treatment complications. Radiotherapy is utilized for numerous malignancies and secondary nodal disease at the plexus, including breast, lung and head and neck carcinomas. Radiotherapy is a common cause of non-traumatic plexus injury with histology showing fibrous tissue sheathing the plexus and underlying neural degeneration. As in other forms of plexopathy, parasthesias dominate the clinical picture, with motor loss less common.1,13,17
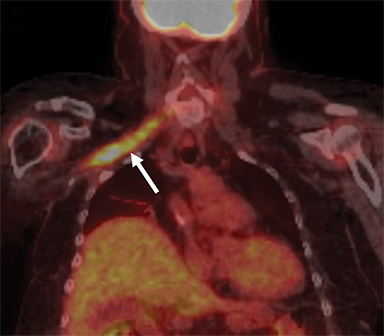
On imaging, it is challenging to differentiate radiation plexopathy from recurrent/residual tumor. MRI of radiation plexopathy in the acute phase (<6months) appears as a non-specific plexopathy with diffuse neural thickening, T2W hyperintensity and mild enhancement (Figure 11). In the more chronic fibrotic phase MRI can demonstrate T2W and T1W hypointense thickening without a focal mass. Enhancement is variable in the fibrotic phase, again making differentiation from tumor challenging. 24 Clinical history is crucial to assess for this entity, with information such as radiation dose (less likely if less than 50Gy) and time course (peak presentation is several years after therapy) especially useful. When doubt remains, early follow-up MRI or additional FDG PET-CT, which can assess for recurrent hypermetabolic tumor, can be utilized.3,25
Benign neoplasms
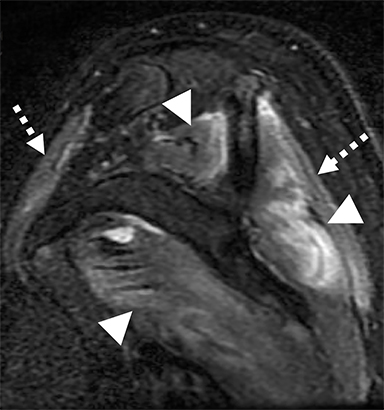
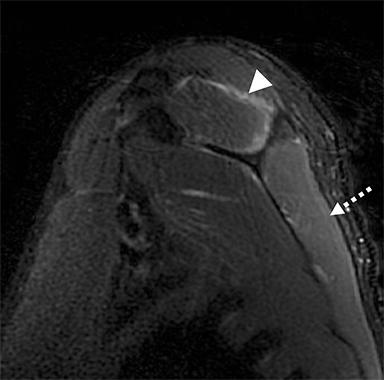
Numerous benign lesions can arise directly from or in the vicinity of the BP. Peripheral nerve sheath tumors (PNST) such as schwannomas and neurofibromas are the most common primary benign lesion of the BP. 26 The majority of PNST are not associated with underlying neurofibromatosis. Patients usually report a painless supraclavicular mass without parasthesia or weakness. A positive Tinel sign may be present. On imaging the lesions are usually well-circumscribed, ovoid (parallel to the course of the BP), with T2W hyperintensity and avid enhancement. A target sign may also be demonstrated (more commonly in neurofibromas) consisting of central low signal and surrounding peripheral T2W hyperintensity (Figure 12). Schwannomas may also demonstrate the salt and pepper fascicular sign, and can undergo degenerative cystic changes (ancient schwannoma).1,26
Other compressive benign lesions arising in the vicinity of the BP include lipomas, fibroproliferative conditions, and vascular lesions (eg hemangioma, arteriovenous malformations or pseudoaneurysms).24
Lipomatous lesions are well characterized on both ultrasound and MRI. On T1W and T2W images the lesions are hyperintense with loss of signal on fat suppression. Differentiating lipomas from low-grade liposarcomas remains difficult, although imaging features such as numerous thick septations, extensive non-fatty components and a large size (>10cm) suggest a more malignant potential.27
Fibroproliferative conditions can present as large masses and include fibromatosis (locally aggressive extra-abdominal desmoid tumor) and nodular fasciitis. The former is typically painless with MRI demonstrating avid enhancement, heterogeneous T2W and hypointense T1W signal and infiltrative margins. In contrast, nodular fasciitis usually presents as a markedly tender mass with similar MR signal characteristics but less marked enhancement. Surgical resection is typically curative in nodular fasciitis, whereas fibromatosis is prone to recurrence following resection and is therefore difficult to treat. For this reason, close MRI follow-up is common in fibromatosis, and may coincide with additional radiation or chemotherapy.1,5,17,28
Brachial plexitis
Inflammation of the BP, termed brachial plexitis, typically presents with acute onset of severe shoulder pain, parasthesias and delayed weakness with muscular atrophy. On clinical follow-up the typical clinical course of pain improvement followed by muscle weakness can differentiate the condition from cervical spondyloarthropathy. The supraspinatus nerve is most commonly affected (~97%) and is involved solely in half of cases.13,17
The cause of brachial plexitis remains uncertain with viral, post-vaccination or auto-immune mechanisms postulated. It is therefore usually termed acute sporadic brachial plexitis or Parsonage-Turner syndrome. 8 A subset of brachial plexitis can occur due to autosomal dominant hereditary neuralgic amyotrophy. Another condition to be aware of is chronic inflammatory demyelinating polyneuropathy, which is rare but classically leads to marked, diffuse thickening of the cervical nerve roots and plexus. 29
MRI is the preferred examination and in the acute phase may show denervation edema in the shoulder girdle musculature, which can progress to fatty atrophy with chronicity (Figure 13). Less commonly, MRI can show nerve root thickening and edema with increased T2W signal.17,24 Contrast administration is not strictly required, but can highlight neural enhancement, indicative of ongoing active inflammation. Practically, the major role of MRI is to exclude a compressive mass, which may require surgical intervention. Ultrasound does not have a major role in brachial plexitis, but may highlight fatty atrophy of the shoulder girdle musculature, and can also exclude compressive lesions; eg, a spinoglenoid notch cyst leading to suprascapular nerve compression.11,13
Infection
Infection of the BP is uncommon and can occur secondary to penetrating trauma or adjacent soft tissue infection, vertebral osteomyelitis, septic arthritis of the glenohumeral joint or direct extension of infection from the lung apex. In patients with suspected infection, especially the immunocompromised, there should be a low threshold for contrast-enhanced MRI. This allows for assessment of infective extent, any adjacent primary source; eg,spinal infection, and for any fluid collections, which could then be targeted for aspiration or surgical debridement. Numerous pathogens are implicated with bacterial infections most common, especially staphylococcus aureus or tuberculosis.1,5,30
Thoracic outlet syndrome
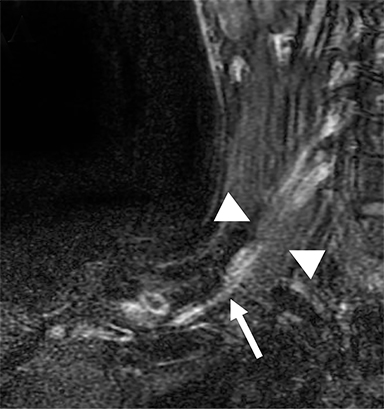
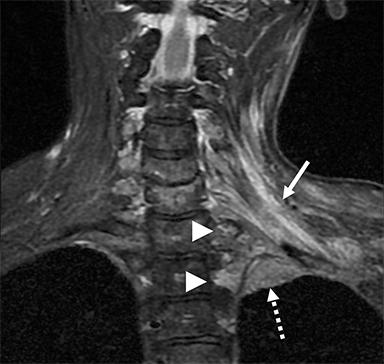
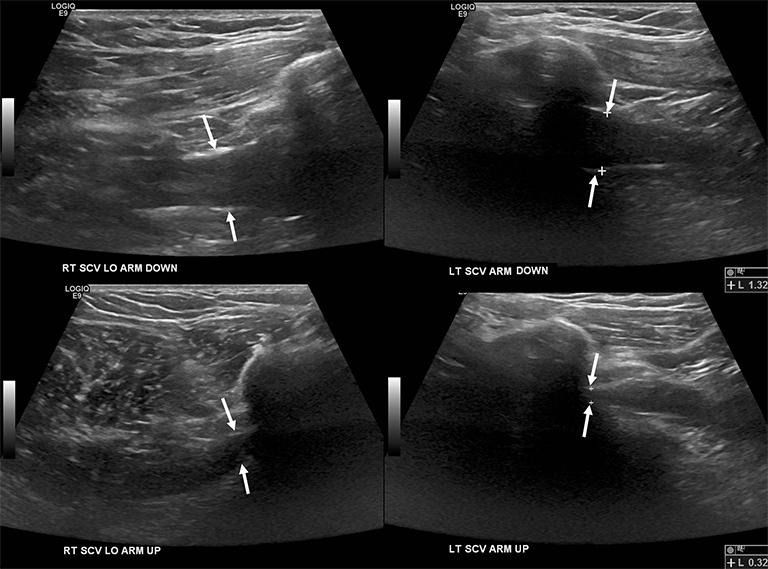
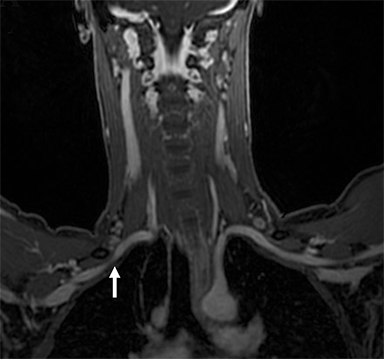
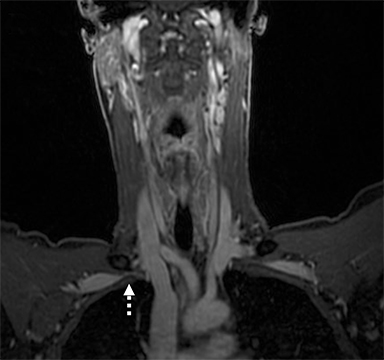
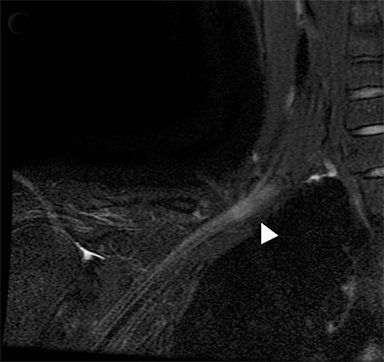
Compression of the subclavian vessels and BP as they traverse the thoracic outlet may produce symptoms such as paresthesia, pain, or swelling. Three potential sites of entrapment exist: interscalene triangle, costoclavicular space (most common site of arterial compression) and the retropectoralis minor space. 8,31 The clinical evaluation of this syndrome is challenging, and provocative ultrasound and MRI can be used in tandem to aid diagnosis. Ultrasound can provide real-time evaluation of vessel stenosis or occlusion using B-mode and Doppler imaging (Figure 14). MRI can then provide high-resolution anatomical detail to highlight the site of compression (Figure 15).8,13
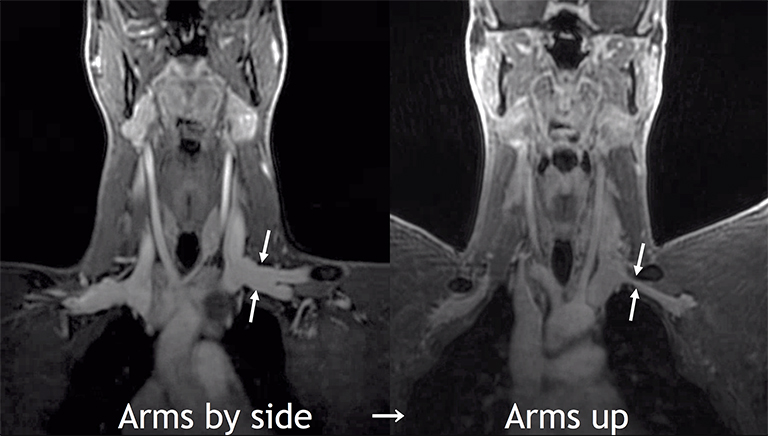
Provocative maneuvers typically involve arm hyperabduction, which accentuates any narrowing at the costoclavicular and retropectoralis minor spaces. The syndrome may result from a variety of causes including a cervical rib, anomalous muscle (eg, subclavius posticus muscle), clavicular fractures with callus formation, fibrous bands or neoplastic lesions.1,17,32,33,34 Diagnosis can be challenging as some degree of venous compression is apparent in most asymptomatic patients on arm abduction.35 In this regard, bilateral imaging may highlight the region of abnormal narrowing on the symptomatic side (Figure 16).
Cervical spondylosis
Due to the vague and non-specific symptoms of BP lesions, it is important to first exclude much more common cervical spondylotic changes. Disc bulges, osteophytes, uncovertebral and facet joint arthropathy can combine to cause cervical cord and/or nerve root compression. Although the cervical spine is covered to some degree on BP protocols, initial dedicated cervical spine MRI may identify the cause of the clinical complaint avoiding the need for the more time intensive BP examination.
Conclusion
The BP can be imaged in detail using dedicated MRI protocols, which allow for accurate interpretation by the radiologist. A dedicated BP protocol should include bilateral coronal and unilateral sagittal sections to identify normal anatomy and highlight pathology. A plethora of lesions occur at the BP, predominantly associated with trauma, but also including primary and secondary neoplasia, inflammation and less commonly infection. Practical reporting should include the site of the BP lesion (pre versus post-ganglionic), involved segment (eg, root, division, etc.), and any associated mass or compressive structure (eg, cervical rib). In the latter, a dedicated thoracic outlet MRI protocol in tandem with Doppler ultrasound can be used to assess dynamic changes in adjacent subclavian vascular caliber and flow.
References
- Mikityansky I, Zager EL, Yousem DM, et al. MR Imaging of the brachial plexus. Magn Reson Imaging Clin N Am. 2012;20(4):791-826.
- Johnson EO, Vekris M, Demesticha T, et al. Neuroanatomy of the brachial plexus: normal and variant anatomy of its formation. Surg Radiol Anat. 2010;32(3):291-297.
- Castillo M. Imaging the anatomy of the brachial plexus: review and self-assessment module. AJR Am J Roentgenol. 2005;185(6 Suppl):S196-204.
- Lury KM, Castillo M. Imaging of the brachial plexus. Appl Radiol. April 13, 2004. https://www.appliedradiology.com/articles/imaging-of-the-brachial-plexus Accessed June 8, 2018
- Vijayasarathi A, Chokshi FH. MRI of the brachial plexus: A practical review. Appl Radiol. 2016;45(4):9-18.
- Chhabra A, Thawait GK, Soldatos T, et al. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol. 2013;34(3):486-497.
- Torres C, Mailley K, O’Donovan R. MRI of the Brachial Plexus: Modified Imaging Technique Leading to a Better Characterization of Its Anatomy and Pathology. Neuroradiol J. 2013; 26(6): 699–719.
- Linda DD, Harish S, Stewart BG, et al. Multimodality imaging of peripheral neuropathies of the upper limb and brachial plexus. Radiographics. 2010;30(5):1373-1400.
- Tagliafico A, Calabrese M, Puntoni M, et al. Brachial plexus MR imaging: accuracy and reproducibility of DTI-derived measurements and fibre tractography at 3.0-T. Eur Radiol. 2011;21(8):1764-1771.
- Martín Noguerol T, Barousse R, Socolovsky M, et al. Quantitative magnetic resonance (MR) neurography for evaluation of peripheral nerves and plexus injuries. Quant Imaging Med Surg. 2017;7(4):398-421.
- Haber HP, Sinis N, Haerle M, et al. Sonography of brachial plexus traction injuries. AJR Am J Roentgenol. 2006;186(6):1787-1791.
- Graif M, Martinoli C, Rochkind S, et al. Sonographic evaluation of brachial plexus pathology. Eur Radiol. 2004;14(2):193-200.
- Lapegue F, Faruch-Bilfeld M, Demondion X, et al. Ultrasonography of the brachial plexus, normal appearance and practical applications. Diagn Interv Imaging. 2014;95(3):259-275.
- Haun DW, Cho JC, Clark TB, et al. Normative cross-sectional area of the brachial plexus and subclavian artery using ultrasonography. J Manipulative Physiol Ther. 2009;32(7):564-570.
- Demondion X, Vidal C, Herbinet P, et al. Ultrasonographic assessment of arterial cross-sectional area in the thoracic outlet on postural maneuvers measured with power Doppler ultrasonography in both asymptomatic and symptomatic populations. J Ultrasound Med. 2006;25(2):217-224.
- Posniak HV, Olson MC, Dudiak CM, et al. MR imaging of the brachial plexus. AJR Am J Roentgenol. 1993;161(2):373-379.
- Panwar JS, Jakkani RK, Thomas BP. TMR Imaging of non-traumatic intrinsic brachial plexus neuropathy: Spectrum of findings. J Nucl Med Radiat Ther. 2015;6(5):246-255.
- Yoshikawa T, Hayashi N, Yamamoto S, et al. Brachial plexus injury: clinical manifestations, conventional imaging findings, and the latest imaging techniques. Radiographics. 2006;26(Suppl 1):S133-143.
- Silbermann-Hoffman O, Teboul F. Post-traumatic brachial plexus MRI in practice. Diagn Interv Imaging. 2013;94(10):925-943.
- Kim DH, Murovic JA, Tiel RL, et al. Infraclavicular brachial plexus stretch injury. Neurosurg Focus. 2004;16(5):1-6.
- Nagano A, Ochiai N, Sugioka H, et al. Usefulness of myelography in brachial plexus injuries. J Bone Joint Surg. 1989;14:59-64.
- Hale HB, Bae DS, Waters PM. Current concepts in the management of brachial plexus birth palsy. J Hand Surg Am. 2010;35(2):322-331.
- Lee JH, Cheng KL, Choi YJ, et al. High-resolution imaging of neural anatomy and Pathology of the Neck. Korean J Radiol. 2017;18(1):180-193.
- Boulanger X, Ledoux JB, Brun AL, et al. Imaging of the non-traumatic brachial plexus. Diagn Interv Imaging. 2013;94(10):945-956.
- Pierce SM, Recht A, Lingos TI, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23(5):915–923.
- Saifuddin A. Imaging tumours of the brachial plexus. Skeletal Radiol. 2003;32(7):375–387.
- Kransdorf MJ, Bancroft LW, Peterson JJ, et al. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002; 224(1):99–104.
- Skubitz KM. Biology and treatment of aggressive fibromatosis or desmoid tumor. Mayo Clin Proc. 2017;92(6):947-964.
- Lozeron P, Lacour MC, Vandendries C, et al. Contribution of plexus MRI in the diagnosis of atypical chronic inflammatory demyelinating polyneuropathies. J Neurol Sci. 2016;360:170-175.
- White HD, White BA, Boethel C, et al. Pancoast’s syndrome secondary to infectious etiologies: a not so uncommon occurrence. Am J Med Sci. 2011;341(4):333-336.
- Vemuri C, McLaughlin LN, Abuirqeba AA, et al. Clinical presentation and management of arterial thoracic outlet syndrome. J Vasc Surg. 2017;65(5):1429-1439.
- Aralasmak A, Karaali K, Cevikol C, et al. MR imaging findings in brachial plexopathy with thoracic outlet syndrome. AJNR Am J Neuroradiol. 2010;31(3):410–417.
- Akita K, Ibukuro K, Yamaguchi K, et al. The subclavius posticus muscle: a factor in arterial, venous or brachial plexus compression? Surg Radiol Anat. 2000;22(2):111-115.
- Forcada P, Rodríguez-Niedenführ M, Llusá M, et al. Subclavius posticus muscle: supernumerary muscle as a potential cause for thoracic outlet syndrome. Clin Anat. 2001;14(1):55-57.
- Demondion X, Boutry N, Drizenko A, et al. Thoracic outlet: anatomic correlation with MR imaging. AJR Am J Roentgenol. 2000;175(2):417-422.
Dr. Hallinan is a Radiologist with the National University Health System, Singapore, and an Assistant Professor of Radiology at the Yong Loo Lin School of Medicine, Singapore; Dr. Pathria and Dr. Huang are Radiologists with the UC San Diego Health System. Dr. Pathria is a Professor, and Dr. Huang is an Associate Professor of Radiology at the San Diego Medical Center, University of California, San Diego, CA. None of the authors have conflicts of interest to declare.
