Automated breast ultrasound: An update
Images







With increasing physician and patient awareness of breast density and its relationship to breast cancer, along with the passage of breast density notification laws by many states, radiologists are considering supplementary ultrasound for breast cancer screening. Automated breast ultrasound (ABUS) received FDA approval1 as an adjunct to screening mammography in patients with dense breasts in 2012 and is expected to overcome the limitations of off-label use of handheld ultrasound for breast screening. With FDA approval of ABUS comes a requirement of eight hours of peer-to-peer training. This article reviews the current status of ABUS and considers the necessity and format of training.
Breast density and risk of breast cancer
Most radiologists report breast density using the American College of Radiology Breast Imaging Reporting and Data System2 (BI-RADS®), which ranges from almost entirely fat (less than 25% glandular) through scattered fibroglandular densities (approximately 25-50% glandular), heterogeneously dense (approximately 51-75% glandular) and extremely dense (more than 75% glandular), based upon a visual determination. Increased breast density is generally defined as breasts with BI-RADS density assessments of 3 and 4 (heterogeneously dense and extremely dense breast tissue). Since the first reports of the association of increased breast density by Wofle3,4 and Tabar,5 published studies on breast density and its relationship to breast cancer risk are now numerous and growing.
Mammographic breast density is an independent risk factor for breast cancer. The degree of risk that is most often quoted is 4-6X.6 Applying this risk to all patients with dense breasts is incorrect and misleading; it is important to put this into the correct context. The “4-6X” increased risk is a result of comparing breast cancer risk in patients with extremely dense breasts to patients with almost entirely fatty breasts ( <10% dense), each representing about 10% of the US population.7 The relative risk is 1.5 or less for the remainder of patients with heterogeneously dense breasts, compared to those with scattered fibroglandular density, which each represent about 40% of the population.7
Patient breast-density awareness increasing
A 2009 Harris poll showed little patient awareness of personal breast density and its relationship to breast cancer risk.8 At that time, >90% of patients were unaware of their breast density and >95% were unaware of increased risk of breast cancer. The first legislation mandating payer coverage for supplementary breast cancer screening for patients with dense breasts, and later mandated density notification by Connecticut in 2009, brought national attention to this issue.9 As of this writing, 15 states have passed similar “density inform” legislation and a federal bill has been introduced (HR 3404).10 In addition, the FDA is considering requiring breast density notification to patients via regulatory change.11
Automated breast density software: Is it necessary?
Some have opined that automated density software is necessary to address concerns of reproducibility of density classification among radiologists,12 while others have questioned the use of 2D mammographic data to assess density of a three-dimensional organ.13 Community radiologists assigning BI-RADS® density classification in a prospective study of over 60,000 patients were able to show a 4.6-fold increased risk of breast cancer for extremely dense breasts compared to fatty breasts.14 Certainly, breast density assessment with automated systems is likely to be more reproducible than radiologists using BI-RADS, but it is not necessary to begin an ultrasound screening program. What is often lost in the discussion about the need for more robust density measurement is that the majority, if not all, of the published ultrasound screening studies (see below), used BI-RADS density assessment to identify those patients to be screened with ultrasound. The masking effect of the density itself is at least as important as the risk of breast cancer in selecting patients to be screened.
Breast ultrasound for screening
Screening mammography is the only detection method shown to reduce mortality from breast cancer in randomized controlled trials. In a review of the longstanding Two Country Trial in Sweden, the initial data showing a reduction in mortality of 30% among those patients “invited” to screening has remained stable.15 However, mammography’s benefit is limited in patients with dense breasts secondary to the masking effect of the density itself.16-18
Prior to 2010, nine published studies using ultrasound to supplement mammography for screening in almost 60,000 patients showed an increased detection rate averaging about 3.5 cancers per thousand.16,17, 19-25 Studies published since have shown similar detection rates. To many breast imagers and referring physicians, it seems clear that some form of supplementary screening is necessary in this population, and because of limitations inherent to screening with handheld ultrasound (HHUS), many radiologists are considering automated breast ultrasound. The hope is that ABUS can both reduce the time to acquire images (average 19 minutes for physician-performed HHUS scans17), as well as reduce operator dependence, a longtime issue associated with HHUS devices.
Two technologies are available for ABUS. One uses a standard-size handheld transducer guided by an automated articulated arm, generating standard 2D images interpreted in a cine format. This exam requires the images to be acquired by a sonographer, although it has potential to limit operator dependence. The only published research showed good sensitivity and specificity.16 The second, competing technology uses a large-format transducer; typically, six 3D volumes are acquired in the transverse plane—three for each breast. This device can be operated by virtually anyone after a three-day, hands-on training period, with potential to reduce sonographer time and operator dependence. The volumetric datasets are immediately sent to a dedicated physician workstation, where the transverse images are reconstructed in coronal and sagittal planes with user-adjustable slice thickness ranging from 0.5mm to 3mm in all three orthogonal planes, as well as radial and anti-radial slicing. Reviewing the exam is, therefore, similar to MRI or CT, with 3D multiplanar datasets and contiguous images (Figure 1).
Callback rate
One criticism of screening breast ultrasound is the potential increase in callback rates and false-positive interpretations. Certainly, recent published experience justifies the criticism. What is an acceptable callback rate when supplementing mammography with US? Many would say “zero”, but this is unrealistic, as no imaging technology has ever achieved perfect sensitivity and specificity. A reasonable compromise would be an increased callback rate that is equivalent to the increase in the cancer detection rate. For example, consider a mammographic cancer detection rate of 5/1000 and a callback rate of 10%: If the cancer detection of supplementary ultrasound is an additional 3/1000, we might accept a screening ultrasound callback rate of 6% (60% increase in overall cancer detection rate).
Two workflows can be implemented for ABUS. The first is to interpret the screening mammogram, and if it is negative and dense, then offer ABUS. Although consistent with the FDA-approved indication for use, this option may require a second appointment for the patient if the mammogram is not interpreted immediately. Effectively recalling all patients with dense breast tissue and normal mammography reduces the acceptance of ABUS.
The second workflow is to offer ABUS immediately after the mammogram, based on density assessment alone. In this author’s experience, this off-label approach greatly increases patient acceptance. A trained radiologic technologist may perform the density assessment, which was the approach utilized by the prospective Somo*Insight Registry Study or, alternatively, computerized density software such as Quantra, Vu-Comp, or Volpara, may be used to triage patients into ABUS and no-ABUS groups. Similar to a mammographer, computerized density software can immediately report the percentage breast density, but these results are objective rather than reliant on the subjectivity of a technologist. If density assessment results yield a percentage of fibroglandular tissue >50% of breast volume, ABUS can be performed after the mammogram and prior to mammographic interpretation. This approach has the potential to offset mammographic callbacks due to cysts, given that benign cysts diagnosed on ABUS would not need a handheld ultrasound scan for diagnosis.
As Laszlo Tabar often teaches in mammography, “screening is not diagnosis,” and this applies to breast ultrasound as well. Screening breast ultrasound requires a paradigm shift in the radiologist’s approach to interpretation. Most radiologists, having not performed screening breast ultrasound, typically approach US screening from a diagnostic perspective. The cancer-detection rate of a screening study is approximately tenfold lower compared with a diagnostic study.26 Given this, the likelihood of malignancy of findings detected with screening breast US that would be called probably benign (BI-RADS® 3) on diagnostic US is much lower, and may not justify recall. A request to develop new criteria to classify benign-appearing solid and cystic masses seen at screening ultrasound has been made.27
FDA premarket approval (PMA)/reader study
U.S. FDA approval is required by a device manufacturer before marketing products such as digital tomosynthesis and ABUS for new indications related to the detection or diagnosis of diseases such as breast cancer. To prove safety and effectiveness, reader studies are the preferred methodology, given the shorter timeframe and cost as opposed to a multiyear study of thousands of patients necessary in a screening population with a low incidence and/or prevalence of disease (eg, breast cancer). The FDA granted premarket approval (PMA)1 on Sept. 18, 2012, to ABUS as an adjunct to mammography for screening in patients with heterogeneously and extremely dense breasts after reviewing results of the Pivotal Clinical Retrospective Reader Study (USI 2011001, Pivotal CRRS).28
In the first day of the reader study, the 17 participating radiologists were given 80 hours of training in interpreting ABUS, given the unique nature of the 3D acquisition and display of the breast images. On days 2 and 3, the readers interpreted 200 cases, most including digital mammograms followed by ABUS. In typical reader study design, the readers were first instructed to give an initial BI-RADS category assessment, followed by a forced BI-RADS (if other than assessment category 1 or 2), likelihood of malignancy from 1-100% (LOM) for up to three lesions in each breast, and finally, BI-RADS assessment and LOM for the entire mammogram. After recording the results, the readers interpreted the ABUS examination in similar fashion. The study goal was to determine if reader performance for breast cancer detection improved when digital mammography as combined with ABUS, compared to using digital mammography alone, in patients with BI-RADS density assessment 3/4 and assigned a BI-RADS assessment category 1/2 (negative/benign). Reader performance was assessed by evaluating area under Reader Operating Characteristic (ROC) Curve (AUC).
The reader study demonstrated a statistically significant improvement in breast cancer detection sensitivity when digital mammography was supplemented with ABUS. Importantly, there was no statistically significant decrease in specificity. Given prior studies showing decreased specificity of screening breast ultrasound, the FDA mandated that radiologists purchasing ABUS be trained substantially similarly to the readers in the Pivotal Clinical Retrospective Reader Study.17 The Pivotal CRRS has been submitted for publication.
Because non-cancer cases with prior breast intervention were not included in the Pivotal CRRS, the FDA approval limits screening ABUS to asymptomatic patients with no prior breast intervention in addition to normal mammography and >50% breast density. For this reason the publicly marketed Pivotal CRRS results represent a sub-analysis of the Pivotal CRRS dataset, which includes only the subset of cases that meet the patient population criteria described by the approved indication for use.
ABUS training specifics
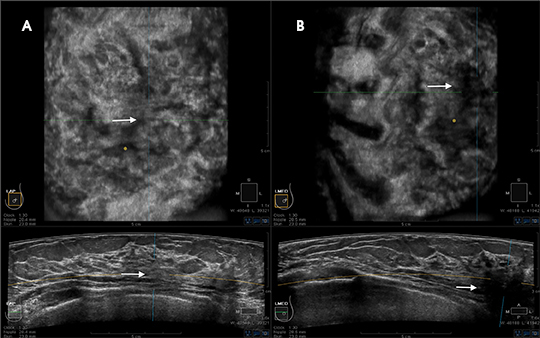
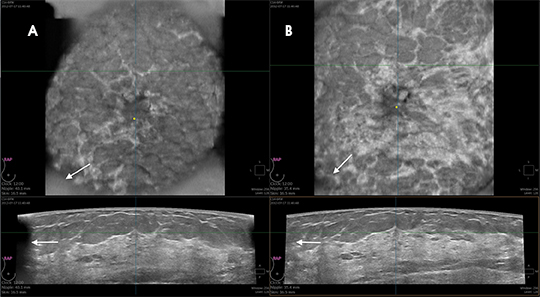
Reader training combines orientation to the unique workstation displaying 3D datasets, including “knobology” and direction on evaluating the 3D datasets in a step-wise logical progression, with an approach to breast cancer screening using ABUS, with the goal of improving sensitivity without negatively affecting specificity (callback rates). Demonstrating live or recorded acquisition of the 3D volume ABUS datasets helps the radiologist appreciate the unique nature of the large-format transducer (Figure 2) and contrasts the sizes of the handheld transducer and the ABUS transducer (Figure 3). Understanding redundant information provided in overlapping 3D datasets improves sensitivity and specificity (Figures 4 and 5). Discussing potential pitfalls of positioning and compression helps the reader gain a better foundation for interpreting ABUS images (Figures 6 and 7).
Training is divided into four modules. Module 1 is a one-hour, peer-to-peer webinar with an initial orientation to ABUS. Module 2 is a three-hour, web-based session of self-paced tutorials of ABUS cases. Modules 3 and 4, which represent the “nuts and bolts” of ABUS interpretation, consist of live peer-to-peer interactive instruction with hands-on ABUS interpretation and assessment of image quality. Modules 3 and 4 may be delivered in-person by a qualified Peer Educator (Figure 8), or remotely through a virtual classroom that utilizes bilateral remote desktop sharing between the Peer Educator and ABUS Learner with live video/voice conferencing. After training is complete, each ABUS learner completes a final self-assessment of 10 cases at his own pace to gauge individual performance. The caseload consists of cancerous, benign, and normal studies. Individual results are provided to the ABUS learner and benchmarked against the performance of his peers (Figure 9).
Conclusion
Growing physician and patient awareness of increased breast-cancer risk due to breast density and associated limitations of mammography have driven breast density notification legislation, as well as research and development of adjunctive screening tools such as automated breast ultrasound.. Given the limitations of scan time and operator dependence of handheld ultrasound for screening, ABUS appears to be a useful alternative. Reader study results are promising and radiologist training is important to maximize sensitivity while maintaining adequate specificity. Published research with ABUS is currently limited. Results of the Somo*Insight Registry Study are pending. Further research with ABUS will be important to developing new criteria for which lesions constitute benign and probably-benign findings, given a lower expected positive predictive value on a screening examination, and to determine its ultimate effectiveness in reducing breast cancer mortality.
References
- “Premarket Approval (PMA).” http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=7828. Accessed Dec. 10, 2013.
- American College of Radiology. Breast Imaging Reporting and Data System. 4th ed. Reston,VA: American College of Radiology; 2003.
- Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer.1976;37:2486-2492.
- Wolfe JN. Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol.1976;126:1130-1137.
- Tabar L., Dean, PB. Mammographic parenchymal patterns: risk indicator for breast cancer? JAMA. 1982;247:185-189.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med.2007;356:227-36.
- Sickles, EA. The use of breast imaging to screen women at high risk for cancer. Radiol Clin North Am.2010;48:859-878.
- The Harris Poll®April 28-30, 2010, Harris Interactive Inc. All rights reserved.
- Dehkordy SF, Carlos RC. Dense breast legislation in the United States: State of the states. J Am Coll Radiol. 2013;10:899-902.
- H.R. 3404--113th Congress: Breast Density and Mammography Reporting Act of 2013. (2013). In www.GovTrack.us. Retrieved December 8, 2013, from http://www.govtrack.us/congress/bills/113/hr3404
- Federal Register: Mammography Quality Standards Act: Regulatory Amendments. Accessed December 8, 2013. Available at: https://www.federalregister.gov/regulations/0910-AH04/mammography-quality-standards-act-regulatory-amendments.
- Chang TS. Much ado about mammographic breast density. Imaging Technology News. 2012;52:30-32.
- Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and cancer risk. Radiology. 2008; 246:348-353.
- Vacek PM, Geller BM. A prospective study of breast cancer risk using routine mammographic breast density measurements. Cancer Epidemiol Biomarkers Prev. 2004;13:715–722.
- Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260:658-663.
- Kelly KM, Dean J, Comulada WS, et al. Breast cancer detection: using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20:734-742.
- Berg WA, Blume JD, Cormack JB, et al: Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151-2163.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA: Cancer J Clin. 2007;57:75–89.
- Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound. A retrospective review. Cancer.1995;76:626–630.
- Buchberger W, DeKoekkoek-Doll P, Springer P, et al. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol.1999;173:921–927.
- Kaplan SS. Clinical Utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001;221:641–649.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175.
- Leconte I, Feger C, Galant C, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003;180:1675–1679.
- Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003;181:177–182.
- Corsetti V, Ferrari A, Ghirardi M, et al. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol med. 2006;111;440–448.
- Sickles EA, Wolverton DE, Dee KE. Performance parameters for screening and diagnostic mammography: specialist and general radiologists. Radiology. 2002;224:861–869.
- Hooley RJ, Greenberg KL, Stackhouse RM, et al. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69.
- Giger EL, et al. “Clinical reader study examining the performance of mammography and automatic breast ultrasound in breast cancer screening.” Submitted for presentation, RSNA 2012.
Citation
Automated breast ultrasound: An update. Appl Radiol.
September 5, 2014