Clival chordoma in a young child
Images





CASE SUMMARY
A 6-year-old boy was brought to the pediatric emergency by his parents with a fortnight’s history of difficulty in swallowing, regurgitation of food and hoarseness of voice, with recent difficulty in breathing. On examination, the child had a weak gag reflex and shallow breathing with decreased respiratory drive. Chest radiograph revealed patchy consolidation and pneumo-atelectatic changes, favoring aspiration pneumonia. Neuroimaging was advised to rule out the central cause of ventilatory failure on clinical findings.
IMAGING FINDINGS
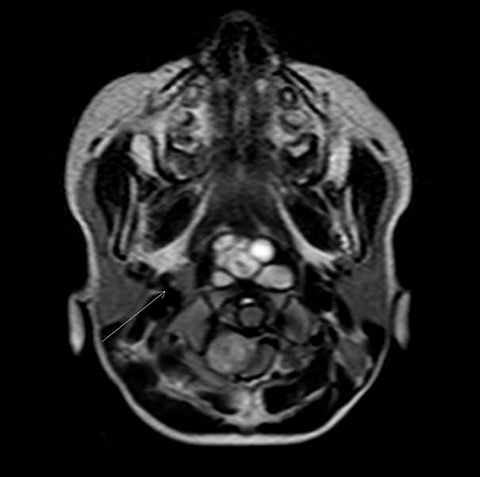
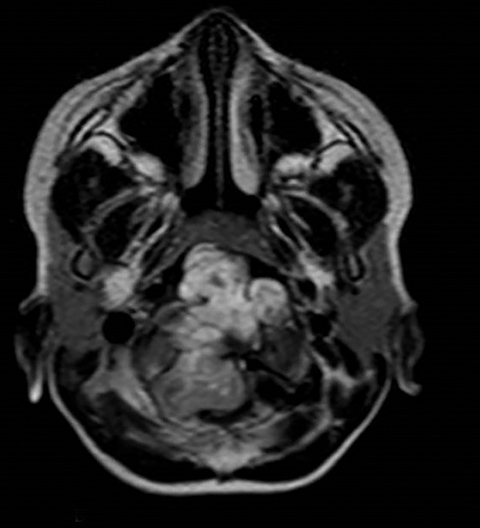
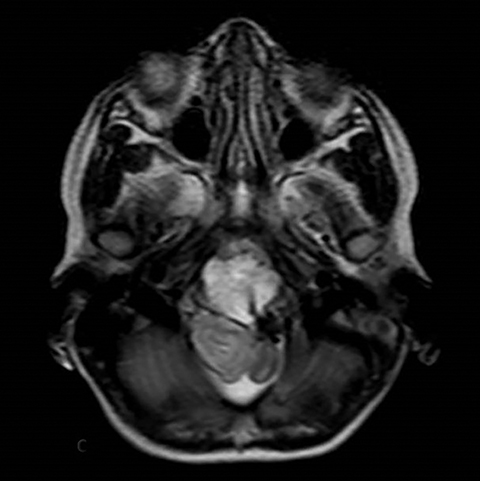
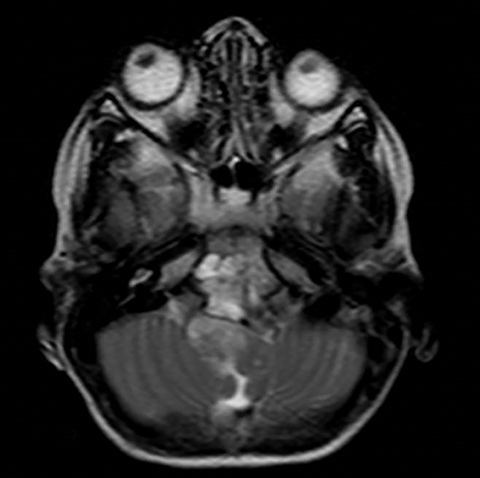
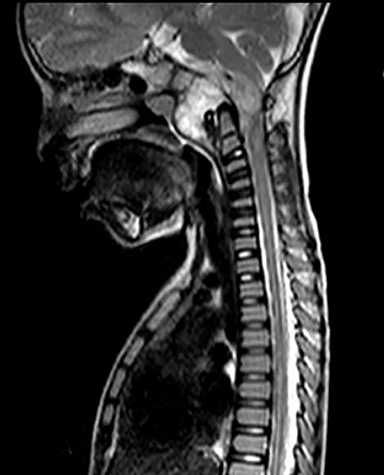
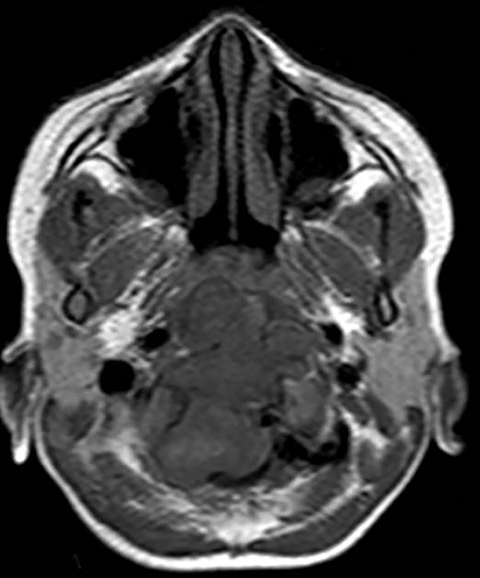
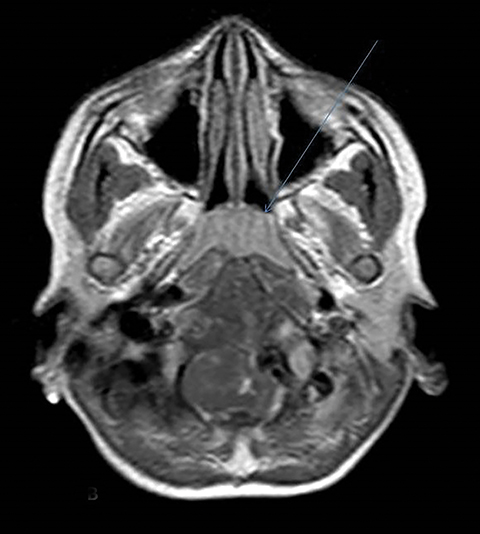
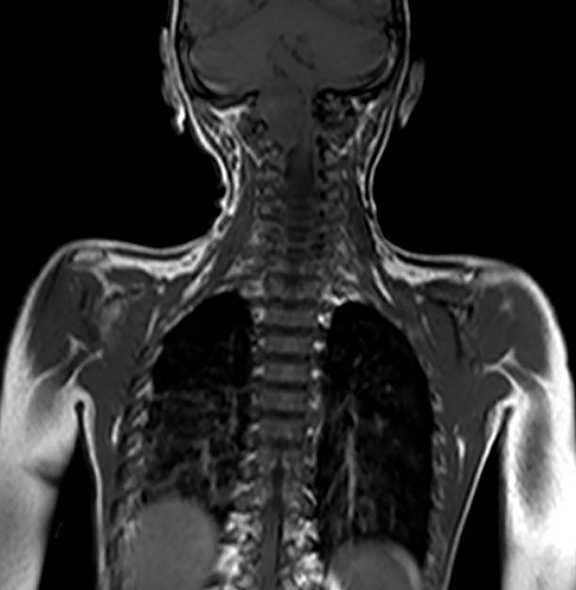
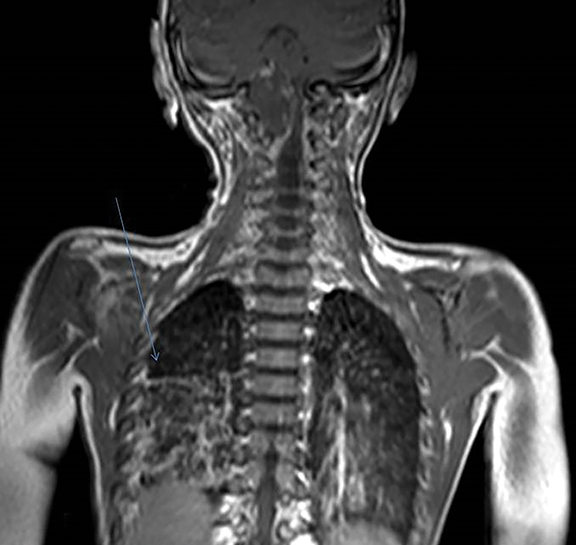
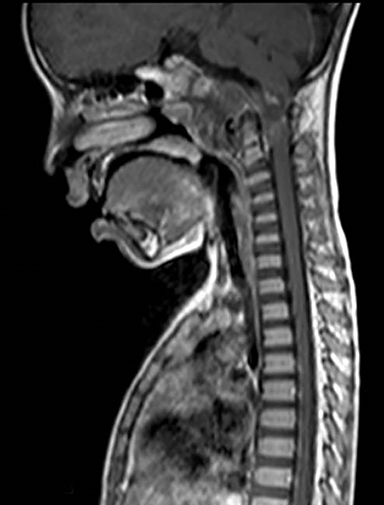
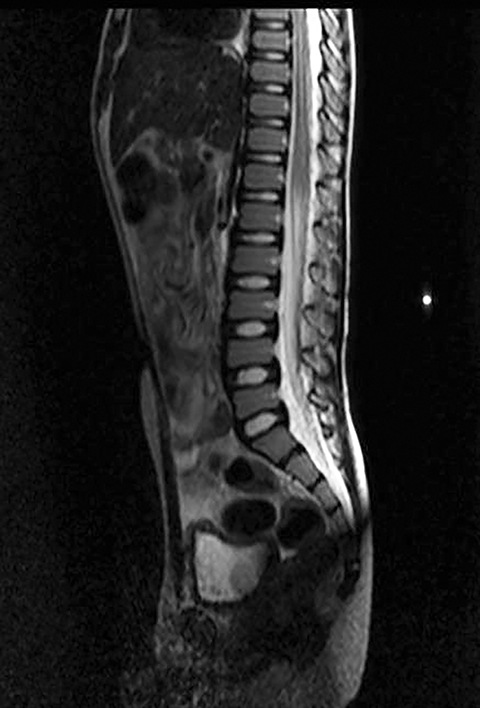
A well-defined lobulated lesion is seen involving the central base of skull with the epicenter in the clivus/Basi- occiput region. On T2-weighted images, it is hyperintense with predominant signals brighter than the intervertebral discs (Figure 1). The lesion shows multiple patchy low signal septations and foci within (Figure 2A). It shows intermediate T1W signals and increased STIR signals (Figure 2B). There is heterogeneous post-contrast enhancement with septal and peripheral enhancement with multiple relatively non-enhancing areas (Figures 3-5). Posteroinferiorly, the lesion is infiltrating the foramen magnum with associated obliteration of anterior thecal sac space and significant extradural compression of the cervicomedullary region of the brain stem, which is seen pushed to the left. The compressed brain stem segment shows increased T2/STIR signals. The proximal cervical spinal cord (up to the level corresponding to the C2-C3 intervertebral disc) appears mildly swollen with hyperintense T2W/STIR signals (cord edema). Screening T2W images of rest of the spine show normal morphology and signal characteristics (Figure 6).
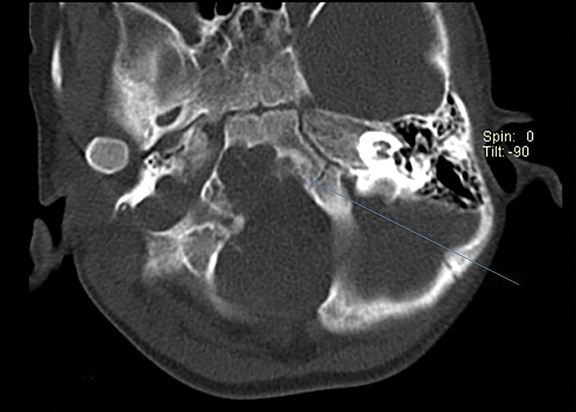
On CT, an expansile osteolytic lesion is seen involving clival occipital bone (basiocciput) with multiple areas of endosteal scalloping and marginal sclerosis, suggestive of chronicity. Involvement of lateral portion of the right occipital condyle, right jugular tubercle of the occipital bone and the hypoglossal canal region is also noted. The lesion shows heterogeneous hypodense soft tissue attenuation with mild and inhomogeneous postcontrast enhancement with patchy areas of low attenuation and soft amorphous calcification within (Figures 7-9).
Biopsy of the lesion done for histopathological examination reveals chondroid component (hyaline cartilage) on histology, suggesting chordoma (chondroid type).
DIAGNOSIS
Clival chordoma (chondroid type) with associated brain stem compression and extrinsic impingement on oropharyngeal airway causing respiratory distress.
DISCUSSION
Chordomas are malignant tumors originating from embryonic notochord remnants in the craniospinal axis, mostly the sacrococcygeal (50 percent) and the spheno-occipital regions (35 percent), though 15 percent can occur in the true vertebrae. The first two sites represent areas where the notochord undergoes complicated folding, thereby incurring greater risk of incomplete involution.
This tumor shows an indolent but an infiltrative locally destructive growth pattern with a high risk of local recurrence.1,2 The mean age at diagnosis is 58.5 years with male predominance,3 with rarely reported cases in children and adolescents.4 Clinical presentation of a clival chordoma depends mainly on the tumor location and the adjacent structures. Though cranial nerve palsies, visual changes, and headache are the most frequent symptoms,5 rarely adults can have CSF rhinorrhoea and epistaxis.6,7
Imaging is useful not only for diagnostic purposes but also for the selection of surgical approaches. Computed tomography (CT) or magnetic resonance imaging (MRI) can identify clival chordoma based on its midline location and destruction of the clivus.8 The typical description of a clival chordoma on CT imaging is that of a well-delineated, hyperdense soft-tissue mass showing moderate-to-marked post-contrast enhancement. Destruction of the clivus is appreciable, which may also give rise to the appearance of intratumoral calcification. On MRI, the tumor will be lobulated, intermediate to hypointense on T1W, and very hyperintense on T2W, secondary to the high fluid content of vacuolated cellular components. Involvement of the clivus will be evident with a “thumb” of tissue indenting the Pons posteriorly. The majority of intracranial chordomas demonstrate moderate-to-marked enhancement following contrast material injection. A distinct advantage of MR imaging is its capacity to display the patency of major vessels as flow voids. MRI can well delineate the internal carotid and basilar arteries and their anatomic relationship to the intracranial chordomas. In spite of high frequency of intracranial arterial involvement, arterial narrowing is rare in intracranial chordomas, a finding reflective of the fact that these tumors are soft and easily dissectible from adjacent vessels.
The three histological subtypes of chordoma are conventional (sometimes called classic) chondroid, and dedifferentiated. Chondroid chordoma usually manifests at a younger age than chondrosarcomas and may have a better prognosis. Chondroid chordomas are relatively less aggressive than conventional chordomas while the dedifferentiated chordomas are more aggressive, faster growing and more likely to metastasize. Close differential diagnosis of a chordoma is a chondrosarcoma.9 Both of them can be similar regarding their location and microscopic appearance. Chondrosarcomas are known to be more responsive to radiation and have a better prognosis.9 The hallmark cell of chordoma is the large physaliphorous cell with a central nucleus and numerous clear cytoplasmic vacuoles. Chondroid chordoma is prominently cartilaginous.
The surgical resection of clival chordomas describes a variety of conventional surgical approaches (transcranial, transsphenoidal, trans-oropharyngeal and maxillary osteotomy).10 Given high local recurrence rate of chordoma, the use of radiotherapy as an adjunct is on the rise to gain local control.
Regardless of the mode of therapy, the local recurrence of intracranial chordomas still occurs irrespective of the optimal treatment.10 MR imaging is the preferred modality for post-surgical follow-up and detection of recurrence. The marked T2 hyperintense signal is more suggestive of tumor recurrence than postoperative changes. Contrast-enhanced images also play a crucial role in making this distinction and in delineating recurrent tumor margins.
CONCLUSION
Intracranial chordoma is a rare and locally aggressive tumor of the skull base that is originating from embryonic remnants of the primitive notochord. CT and MR imaging play an undisputable role in the evaluation of intracranial chordomas, in particular, the osseous involvement and the proximity of these tumors to the critical soft-tissue structures. Combination therapy of radical surgical resection and proton beam radiation therapy achieves the best results. The possibility of a chondroid variety of chordoma should be a differential in a young patient with locally aggressive central skull base lesion, especially with features of scalloping and remodeling.
REFERENCES
- Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: The nonsarcoma primary bone tumor. Oncologist. 2007;12(11):1344-1350.
- Erdem E, Angtuaco EC, Van Hemert R, Park JS, Al-Mefty O. Comprehensive review of intracranial chordoma. Radiographics. 2003;23(4):995-1009.
- McMaster M, Goldstein A, Bromley C, Ishibe N, Parry D. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12(1):1-11. doi: 10.1023/A:1008947301735.
- Menezes A. Craniovertebral junction neoplasms in the pediatric population. Childs Nerv Syst. 2008;24(10):1173-1186.
- Michele SM, Samuel CL: Chordomas of the skull base: manifestations and management. Curr Opin Otolaryngol Head Neck Surg. 2003;11:324-327.
- Kitai R, Yoshida K, Kubota T, Sato K, Handa Y, Kasahara K, Nakajima H: Clival chordoma manifesting as nasal bleeding. A case report. Neuroradiology. 2005;7:368-371.
- Macdonald RL, Cusimano MD, Deck JH, Gullane PJ, Dolan EJ: Cerebrospinal fluid fistula secondary to ecchordosis physaliphora. Neurosurgery. 1990;26:515-519.
- Handa J, Suzuki F, Nioka H, Koyama T. Clivus chordoma in childhood. Surg Neurol. 1987;28(1):58-62.
- Rosenberg AE, Nielsen GP, Keel SB, Renard LG, Fitzek MM, Munzenrider JE, Liebsch NJ. Chondrosarcoma of the base of the skull: A clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. The American Journal of Surgical Pathology. 1999;23(11):1370-1378.
- Hong Jiang W, Ping Zhao S, Hai Xie Z, Zhang H, Zhang J, Yun Xiao J. Endoscopic resection of chordomas in different clival regions. Acta Otolaryngol. 2009;129(1):71-83.
Citation
A C, VV A, PS S, N S, S N, R G. Clival chordoma in a young child. Appl Radiol. 2017;(11):37-41.
November 13, 2017