Cystic Lesions of the Head and Neck: Benign or Malignant?
Images










Editor’s note: This is the first part of a two-part series. Part 2 will appear in the November/December 2020 issue of Applied Radiology.
Cystic lesions of the head and neck, ranging from benign and incidental cysts to life-threatening infections and malignancy, present a common and important diagnostic challenge.
Although some pathologies can present as trans-spatial masses, most cystic lesions are confined to well-defined anatomical spaces. A differential diagnosis can be sharpened by identifying the involved spaces and obtaining a good patient history (Table 1).
This series presents an overview of benign and malignant cystic lesions of the head and neck, emphasizing their appearance on CT and MRI. Part 1 focuses on lesions of the oral cavity, pharynx, masticator space, and parotid space. Part 2 will cover lesions of the carotid space and associated lymph nodes, as well as the retropharyngeal, prevertebral, and visceral spaces, and the supraclavicular fossa.
Lesions of the Oral Cavity
The oral cavity can be divided into 4 anatomical subunits: the oral mucosal surface (or space), the oral tongue, the sublingual space, and the submandibular space.1 Lesions of the oral mucosal surface are readily examined by the clinician and generally not imaged unless a deep component is suspected.
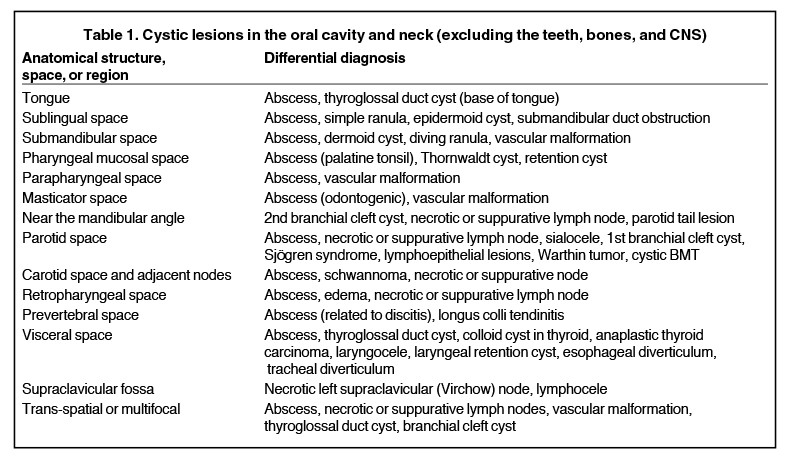
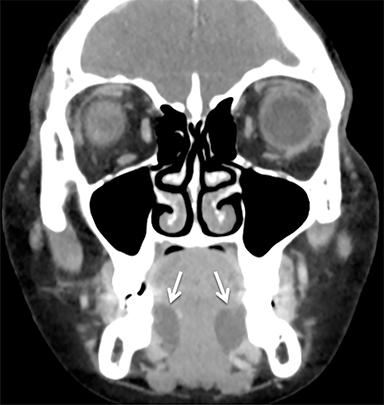
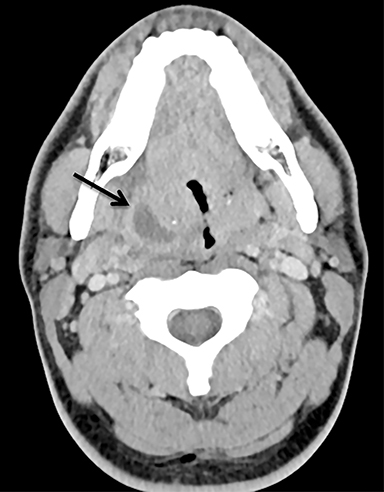
In the oral tongue, the common cystic lesion is a lingual abscess. Similar to abscesses elsewhere in the body, a lingual abscess typically demonstrates a well-circumscribed margin, fluid attenuation on CT or fluid signal intensity on MRI, and peripheral enhancement (Figure 1).2,3 Risk factors include poor oral hygiene, penetrating trauma (eg, piercings), and an immunocompromised state.4
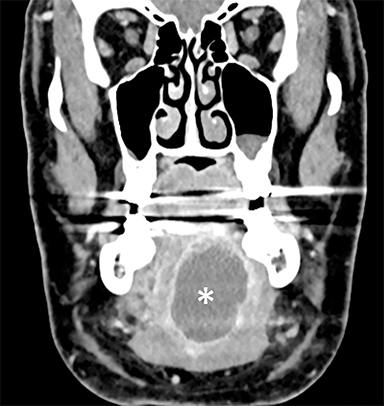
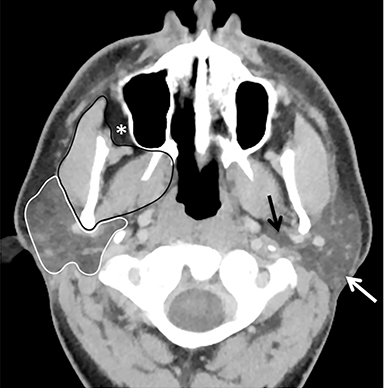
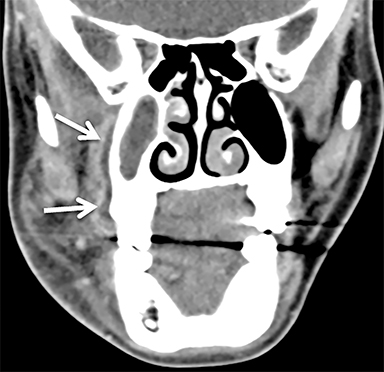
The sublingual space (SLS) lies superior to the mylohyoid muscle and contains fat, the sublingual glands, the submandibular ducts, and neurovascular bundles. The submandibular space (SMS) lies inferior to the mylohyoid muscle and contains fat, the submandibular glands and lymph nodes, and the anterior bellies of the digastric muscles (Figure 2). Both spaces are shaped like a horseshoe, and they communicate at the posterior margin of the mylohyoid muscle.1
The differential diagnoses for cystic lesions in the SLS and SMS are overlapping but not identical. Both spaces can become infected with an abscess. Epidermoid cysts are more common in the SLS, and dermoid cysts (with pathognomonic floating fat globules resembling a “sack of marbles”)5-7 are more common in the SMS.8 Submandibular duct obstruction occurs in the SLS, and may be caused by calculi, which can usually be identified on CT,6 or by strictures, which are better evaluated with conventional or MR sialography.9
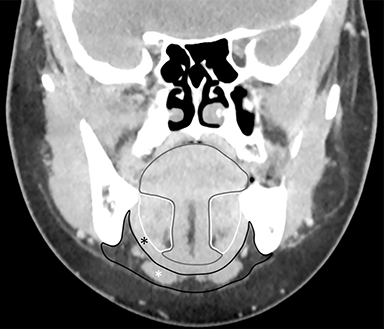
A ranula is a mucinous cyst confined to the SLS, resulting from obstruction or damage to the sublingual gland, or obstruction of the submandibular duct. Unless infected, a ranula is thin walled and unilocular, filling one or both sides of the SLS “horseshoe.”(Figure 3).1,8 An epidermoid cyst can mimic a ranula but is less common. When the ranula extends into the SMS, most commonly via a defect in the mylohyoid muscle,10 it becomes a diving (or plunging) ranula, often leaving only a small residual collection (or “tail”) of fluid in the SLS that is helpful for diagnosis (Figure 4).8,11,12
Vascular malformations can occur almost anywhere in the head and neck and are usually trans-spatial, but they often involve the SMS when they first present in adults.8 These lesions are classified as low-flow and high-flow lesions;13 the low-flow lesions — venous and lymphatic malformations — are most likely to appear cystic on imaging. When a low-flow vascular malformation is suspected, MRI is the preferred modality for evaluating the extent of the lesion. A typical MRI appearance is a trans-spatial, multilocular mass with fluid-fluid levels (Figure 5).14,15 In a venous malformation, portions may appear more solid or microcystic, and phleboliths may be present.
Lesions of the Pharynx
The pharynx is divided into the nasopharynx (posterior to the nasal cavity), oropharynx (posterior to the oral cavity), and hypopharynx (posterior to the larynx). The pharynx is lined by the pharyngeal mucosal space, which includes the mucosal surface, lymphatic tissue (adenoidal, lingual, and palatine tonsils), and submucosal minor salivary glands.1 Many cystic lesions seen in the pharyngeal mucosal space are almost always incidental and asymptomatic.
A Tornwaldt cyst is a notochordal remnant located in the nasopharynx at the midline. Retention cysts of the pharyngeal mucosal space are seen in the nasopharynx (off midline) and in the oropharynx. Both cysts are well circumscribed and thin walled, with the standard imaging features of a simple cyst. The fluid may demonstrate high T1 signal if it is proteinaceous.
A more serious cystic lesion in the pharyngeal mucosal space is the peritonsillar abscess (PTA). The typical appearance on CT is a rim-enhancing fluid collection just deep to an enlarged palatine tonsil (Figure 6).16 In severe cases, pus can extend into adjacent spaces, notably the parapharyngeal, masticator, and submandibular spaces. Rarely, an abscess may form within the parenchyma of the tonsil, referred to as an intratonsillar (or tonsillar) abscess (ITA). On imaging, an ITA is surrounded by tonsillar tissue, distinguishing it from a PTA. Although the distinction between a PTA and ITA is not always clear, it may be helpful for optimizing treatment.17 In any case, the radiologist should provide a precise description of the size and extent of the abscess, including the involved spaces and structures.
Lesions of the Masticator Space and Mandibular Angle
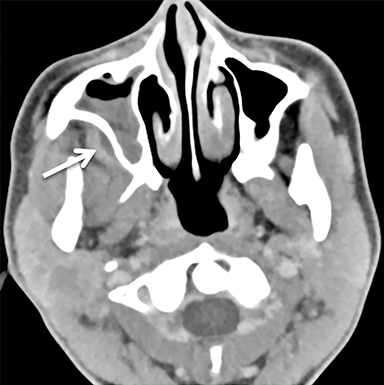
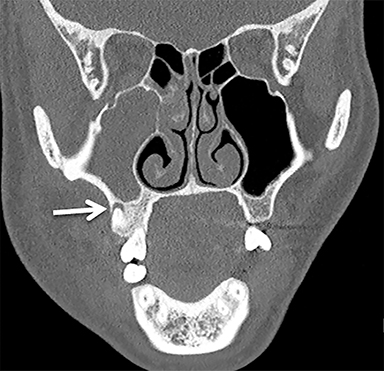
The masticator space is a large paired space containing primarily the muscles of mastication and associated nerves and blood vessels (Figure 7). By far the most common cystic lesion encountered in this space is the odontogenic abscess.1 The source of the infection is usually a mandibular molar or recent dental procedure. Small fluid collections adjacent to the alveolar ridge may be difficult to see on CT, especially if there is streak artifact from dental amalgam, so clinical suspicion is helpful (Figure 8).
Larger abscesses can track along the mandible or maxilla into deeper, more posterior portions of the masticator space, and even extend into adjacent spaces, such as the parapharyngeal or retropharyngeal space. On the bone window/algorithm (Figure 8), CT often shows signs of associated periodontal disease (periapical lucency) and osteomyelitis (cortical dehiscence, permeative/destructive bone changes, periosteal reaction).16
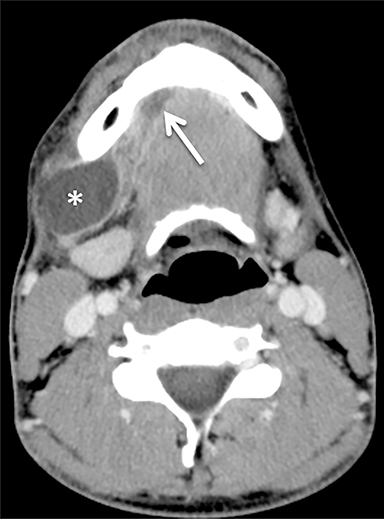
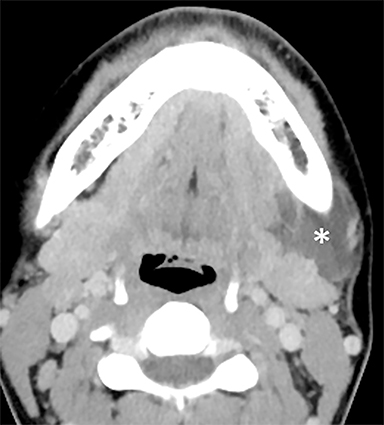
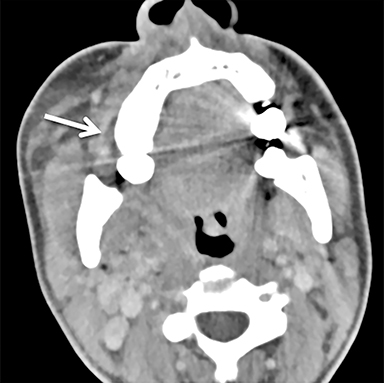
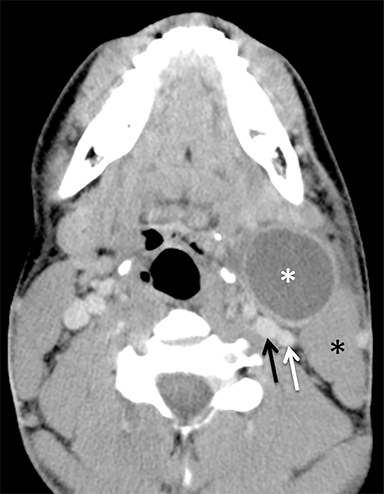
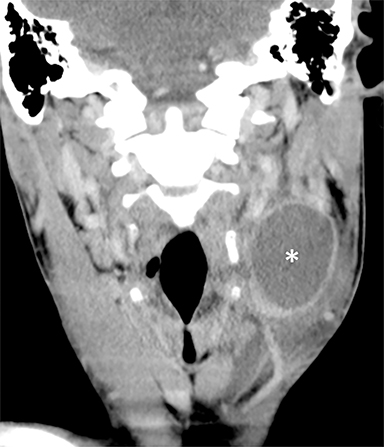
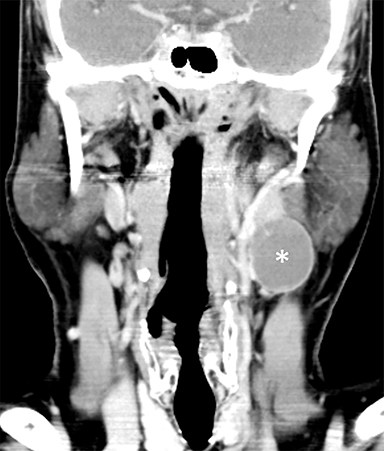
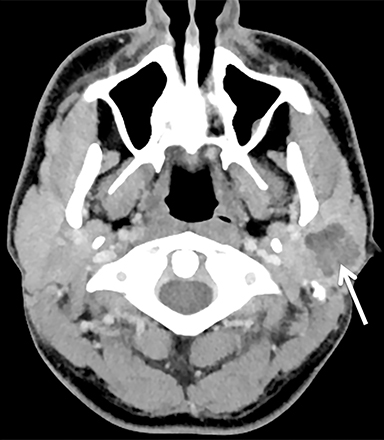
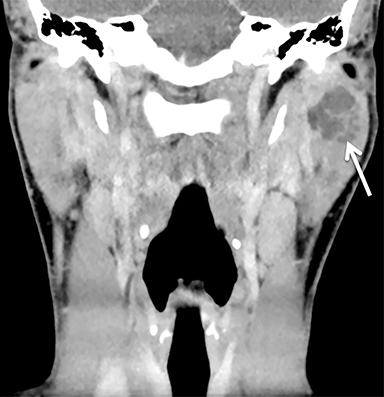
The mandibular angle is a convenient landmark for an important differential diagnosis (Figure 9). In adults, a round cystic lesion at the angle of mandible, just anterior to the sternocleidomastoid, may be a second branchial cleft cyst (2nd BCC; Figure 10) or a level II necrotic lymph node (Figure 11).
The wall of the lesion may be thin (typical for BCCs but also seen in necrotic nodes) or thick and enhancing (more typical for a necrotic node but also seen in infected BCCs). The distinction is important: a 2nd BCC is a benign congenital lesion, whereas a necrotic lymph node may be the first manifestation of oropharyngeal squamous cell carcinoma (SCCa). One clue may be the patient’s age: a 2nd BCC usually presents in early adulthood (age 20-40 years), and oropharyngeal SCCa — even HPV-associated cancer — usually presents after age 40.18
Nevertheless, there is some age overlap, and any new cystic lesion near the mandibular angle in an adult should be viewed with suspicion.19 Although the primary tumor is often small and difficult to see on CT, careful inspection of the palatine tonsils and base of tongue may be fruitful. It is also essential to confirm that the cystic lesion near the mandibular angle does not, in fact, belong to the parotid tail.
Lesions of the Parotid Space
The parotid space contains the parotid gland, the branches of the facial nerve, and the external carotid artery, as well as the retromandibular vein and lymph nodes (Figure 7). The parotid gland wraps around the posterior margin of the mandibular ramus and is often described on cross-sectional imaging as having a deep lobe and a superficial lobe, separated by the retromandibular vein.
The most inferior part of the superficial lobe is referred to as the parotid tail, which can extend to or below the angle of the mandible (Figure 9). The parotid duct courses anterior to the gland superficial to the masseter muscle and terminates near the second maxillary molar.1
A wide variety of benign and malignant cystic lesions can arise in the parotid space, most with overlapping imaging features. Often the differential diagnosis can be narrowed by the clinical history and by whether the lesions are solitary or multifocal.20 A solitary rim-enhancing cystic lesion likely represents an abscess if the parotid gland is enlarged (parotitis) with adjacent fat stranding (cellulitis), and infection is suspected clinically. A large parotid duct with an intraluminal stone suggest the abscess is a complication of sialolithiasis.
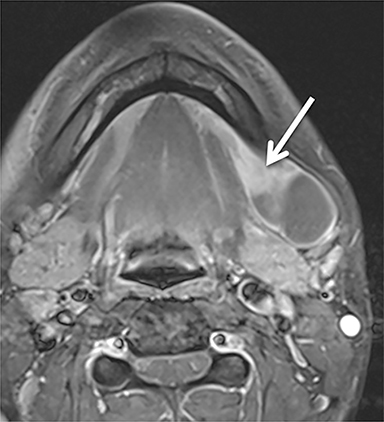
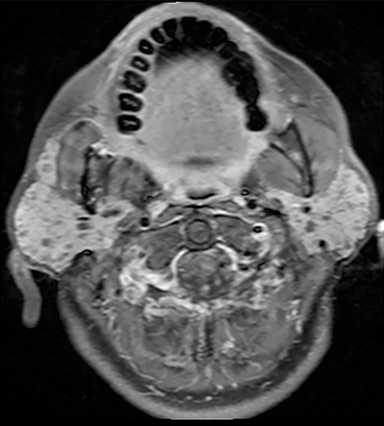
A recurrent parotid abscess raises the possibility of an infected first branchial cleft cyst (1st BCC).5 In the setting of prior trauma, a simple parotid cyst could represent a sialocele. Multiple small, bilateral cysts (Figure 12) represent only two possibilities: Sjögren syndrome (usually a known clinical diagnosis) or benign lymphoepithelial lesions (which can also be solid or mixed cystic and solid) in a patient with human immunodeficiency virus (HIV).21
A cystic mass without associated inflammatory changes in an asymptomatic smoker is suggestive of a papillary cystadenoma lymphomatosum (Warthin tumor), especially if accompanied by multiple or bilateral masses, or if the lesion is located in the parotid tail (the classic “earring lesion”22). However, almost any parotid mass can appear cystic or necrotic, including benign mixed tumor (Figure 13), various carcinomas, and metastatic nodal disease.23
An exact diagnosis is often impossible when evaluating a cystic parotid mass; the more important role of the radiologist is to identify any suspicious features, such as invasive margins, perineural tumor spread, and T2 hypointensity of the solid component (suggestive of highly cellular tumors).24 Because parotid malignancy often mimics benign tumors, the usual management of a parotid mass is surgical.25
References
- Koch BL, Hamilton BE, Hudgins PA, Harnsberger HR. Diagnostic imaging. Head and neck. Third edition. ed. Philadelphia, PA: Elsevier; 2017.
- Fang WS, Wiggins RH, Illner A, et al. Primary lesions of the root of the tongue. Radiographics. 2011;31(7):1907-1922.
- Ozturk M, Mavili E, Erdogan N, Cagli S, Guney E. Tongue abscesses: MR imaging findings. AJNR Am J Neuroradiol. 2006;27(6):1300-1303.
- Srivanitchapoom C, Yata K. Lingual Abscess: Predisposing Factors, Pathophysiology, Clinical Manifestations, Diagnosis, and Management. Int J Otolaryngol. 2018;2018:4504270.
- Wong KT, Lee YY, King AD, Ahuja AT. Imaging of cystic or cyst-like neck masses. Clin Radiol. 2008;63(6):613-622.
- Patel S, Bhatt AA. Imaging of the sublingual and submandibular spaces. Insights Imaging. 2018;9(3):391-401.
- Dillon JR, Avillo AJ, Nelson BL. Dermoid Cyst of the Floor of the Mouth. Head Neck Pathol. 2015;9(3):376-378.
- La’porte SJ, Juttla JK, Lingam RK. Imaging the floor of the mouth and the sublingual space. Radiographics. 2011;31(5):1215-1230.
- Koontz N, Kralik S, Fritsch M, Mosier K. MR siaolography: a pictorial review. Neurographics. 2014;4(3):142-157.
- Lee JY, Lee HY, Kim HJ, et al. Plunging Ranulas Revisited: A CT Study with Emphasis on a Defect of the Mylohyoid Muscle as the Primary Route of Lesion Propagation. Korean J Radiol. 2016;17(2):264-270.
- Kurabayashi T, Ida M, Yasumoto M, et al. MRI of ranulas. Neuroradiology. 2000;42(12):917-922.
- Coit WE, Harnsberger HR, Osborn AG, Smoker WR, Stevens MH, Lufkin RB. Ranulas and their mimics: CT evaluation. Radiology. 1987;163(1):211-216.
- Wassef M, Blei F, Adams D, et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics. 2015; 136(1):e203-214.
- Meesa IR, Srinivasan A. Imaging of the oral cavity. Radiol Clin North Am. 2015;53(1):99-114.
- Gaddikeri S, Vattoth S, Gaddikeri RS, et al. Congenital cystic neck masses: embryology and imaging appearances, with clinicopathological correlation. Curr Probl Diagn Radiol. 2014;43(2):55-67.
- Capps EF, Kinsella JJ, Gupta M, Bhatki AM, Opatowsky MJ. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics. 2010;30(5):1335-1352.
- Ali SA, Kovatch KJ, Smith J, et al. Predictors of intratonsillar versus peritonsillar abscess: A case-control series. Laryngoscope. 2019;129(6):1354-1359.
- Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus-Associated Cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
- Hudgins PA, Gillison M. Second branchial cleft cyst: not!! AJNR Am J Neuroradiol. 2009;30(9):1628-1629.
- Kuan EC, Mallen-St Clair J, St John MA. Evaluation of Parotid Lesions. Otolaryngol Clin North Am. 2016;49(2):313-325.
- Yousem DM, Kraut MA, Chalian AA. Major salivary gland imaging. Radiology. 2000;216(1):19-29.
- Hamilton BE, Salzman KL, Wiggins RH, Harnsberger HR. Earring lesions of the parotid tail. AJNR Am J Neuroradiol. 2003;24(9):1757-1764.
- Kessler AT, Bhatt AA. Review of the Major and Minor Salivary Glands, Part 2: Neoplasms and Tumor-like Lesions. J Clin Imaging Sci. 2018;8:48.
- Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H. MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol. 2011;32(7):1202-1207.
- Carlson ER, Webb DE. The diagnosis and management of parotid disease. Oral Maxillofac Surg Clin North Am. 2013;25(1):31-48.
Citation
B W, III WR. Cystic Lesions of the Head and Neck: Benign or Malignant?. Appl Radiol. 2020;(5):18-24.
September 1, 2020