Distinguishing synovial sarcoma from benign and malignant mimics: MR Imaging indicators
Images






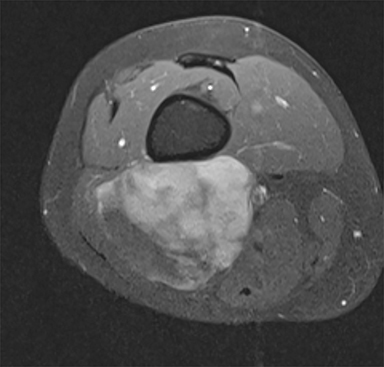
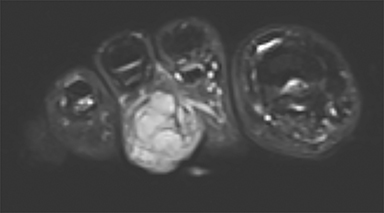

Synovial sarcomas (SS) are malignant soft tissue tumors thought to account for 5-10% of soft tissue sarcomas.1-3 Synovial sarcomas are rare, with an estimated incidence of 2.75 in 100,000 people.4 However, they are the second-most prevalent soft-tissue tumors after rhabdomyosar coma in children, adolescents, and young adults.1
Since synovial sarcomas are so rare, many clinicians and radiologists are not familiar with their presentation and imaging appearance. For these tumors to be biopsied, it is essential that interpreting radiologists or orthopedic oncologists not deem them benign. Incorrectly assuming that an SS is a benign lesion will lead to delays in diagnosis and possibly prognosis. Conversely, mistaking a benign lesion for an SS may result in increased patient anxiety and costs due to unnecessary procedures and imaging tests.
The aim of this article is to describe the key magnetic resonance imaging (MRI) features of SS, as well as to describe those of benign and malignant tumors that are often confused with SS (Table 1).
In one study, 84% of synovial sarcoma patients were between 10 and 50 years old.5 Males and females seem to be equally affected.6 Ninety percent of SS occur in the lower extremities often near joints, and in the trunk wall, but the disease can be found anywhere in the body9 with rare reports of intra-articular SS.10 Synovial sarcomas are high-grade (II/III).11 There are three common histologic types.12 The monophasic type is most common and made up of only of spindle cells.12 The biphasic type consists of spindle cells and epithelial cells.12 Finally, the undifferentiated type is made up of cells resembling those of small round blue cell tumors.12
Factors that result in a worse prognosis include large tumor size, metastases at diagnosis, trunk disease, and positive margins after surgical resection.13 Five-year and 10-year survival rates of SS are estimated to be about 70%, and 60% respectively.13 The poor survival rate of SS is likely related to local recurrence and lung metastases.13
Synovial sarcomas are usually first detected as painless growing masses.14 Confirmation of the presence of a mass by radiography and MRI is usually performed prior to biopsy.14 Definitive SS diagnosis is based on biopsy. The biopsied tissue is often tested for the t(X;18)(p11;q11) translocation using fluorescence in situ hybridization (FISH), for which about 90% of SS are positive.15
MRI: Benign mimickers of synovial sarcomas
Neurofibromas
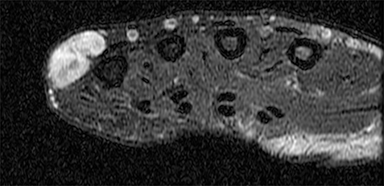
Neurofibromas are benign nerve sheath tumors21 and may be associated with type 1 neurofibrosis (NF1). Neurofibromas usually occur superficially anywhere on the body.22,23 MR imaging features that help identify neurofibromas include (1) arising along a major nerve, (2) visualization of the nerve entering and exiting the tumor, (3) the target sign (round hypointense lesion with hyperintense rim) on T2-weighted imaging (Figure 2), (4) the fascicular sign (multiple small hypointense foci scattered within a hyperintense region on T2-weighted or proton-density weighted MR images), and (5) the split-fat sign—a rim of fat surrounding the lesion (best appreciated on T1-weighted images).24,25 While most neurofibromas show avid enhancement on T1 postcontrast imaging, the enhancement pattern can be variable.26
Hemangiomas
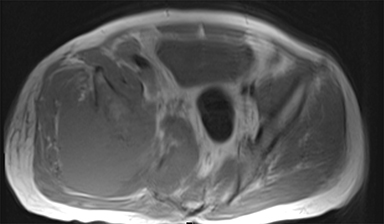
Hemangiomas are an abnormal proliferation of blood vessels and are perhaps better termed venous malformations.27 They are one of the most common soft-tissue tumors in children.28 Hemangiomas affect females three times more often than males.29 The majority of hemangiomas occur superficially, and in the head and neck region.28 On MRI, hemangiomas demonstrate low to intermediate signal intensity on the T1-weighted images; however, they appear hyperintense on T2-weighted images.30,31 They exhibit variable enhancement on T1-weighted images with contrast, depending on flow dynamics and timing of injected contrast bolus.32 Synovial hemangiomas present with the above features, and may also present with low intensity linear structures, likely due to fibrous septa or vascular channels (Figure 3), as well as fluid-fluid levels.33 Because SS can occur intra-articularly and because SS may have fluid-fluid levels, they may be confused with synovial hemangiomas.
Spindle cell lipomas
Spindle cell lipomas are lipomas made up of lipocytes and spindle cells.34 They occur most frequently in males between the ages of 45 and 65, and tend to involve the posterior neck and shoulder.35-39 They are typically superficial and often appear in subcutaneous fat.40 The signal intensity of the nonadipose areas of these lesions is greater than that of fat on T2-weighted sequences, and similar to that of skeletal muscle on T1-weighted sequences.40 The nonadipose tissue displays enhancement, equal to that of nearby vessels after administration of intravenous contrast.40
Pigmented villonodular synovitis (PVNS)/Giant Cell tumors of tenosynovial origin
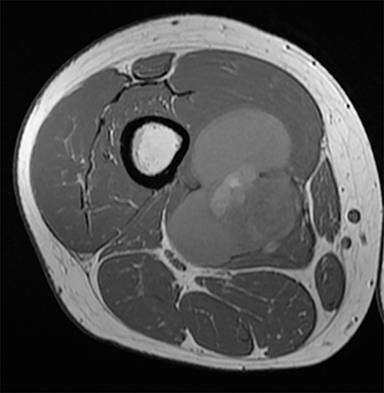
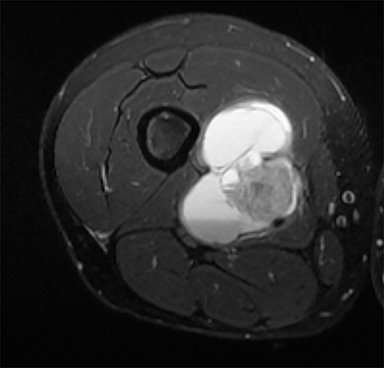
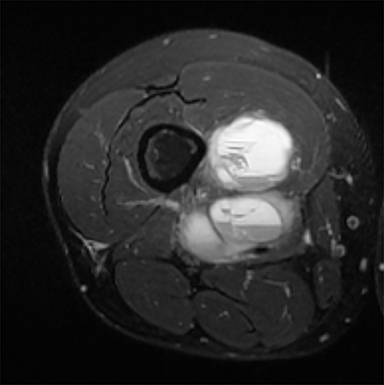
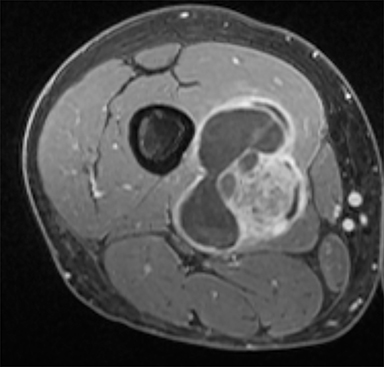
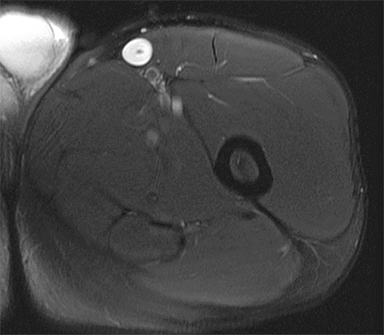
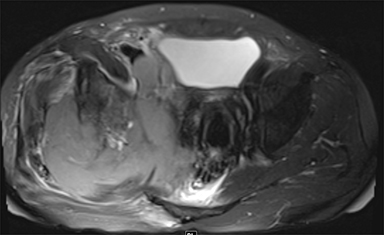
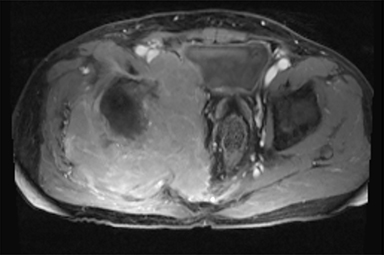
PVNS is a benign and rare monoarticular disease occurring in the joints, characterized by a proliferation of synovial cells, effusions and bone erosions.41 PVNS usually occurs between the 3rd and 5th decades of life.42 PVNS more often involves the knee joint of older individuals, whereas in younger patients PVNS tends to involve the hip.43 The female to male ratio is estimated to be 1.35:10.44 PVNS can be diffuse or localized.45, 46 PVNS shows intermediate signal on T1-weighted images (Figure 4A), low signal on T2-weighted images with areas of high signal (Figure 4B) due to inflamed synovium or joint fluid and variable enhancement on contrast-enhanced T1-weighted images.45, 46 PVNS has low signal on gradient-echo (GE) sequences and sometimes blooming artifacts.45, 46 The blooming artifacts noted on gradient-echo sequences are due to hemosiderin from chronic hemorrhage within these lesions.45,46
Morel-Lavallée lesions
Morel-Lavallée lesions occur post-traumatically and are deep subcutaneous closed degloving injuries where the skin and subcutaneous tissues are separated from the underlying fascia.47 This injury creates a cavity that can fill with blood and necrotic fat.48, 49 The lesions classically occur over the greater trochanter, but can also be seen in the lumbar region, over the scapula and over the knee.50, 51 On MR imaging, the fluid within the cavity can exhibit varying signal intensity and can present with a fluid-fluid levels.50 If the lesion is in the characteristic location with a history of trauma and has the classical appearance, then no differential diagnosis should exist. If not, the lesion can be mistaken for sarcoma.50, 52 If the provided clinical history is equivocal, then short-term follow-up imaging could be used to ensure resolution/change in size of the lesion.
Synovial chondromatosis
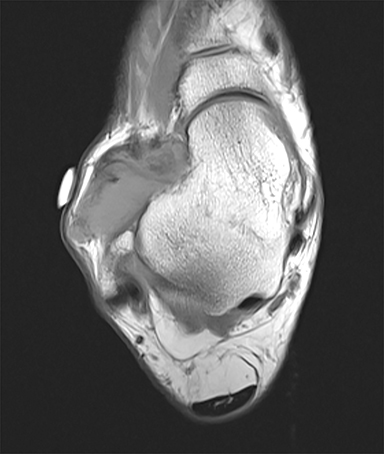
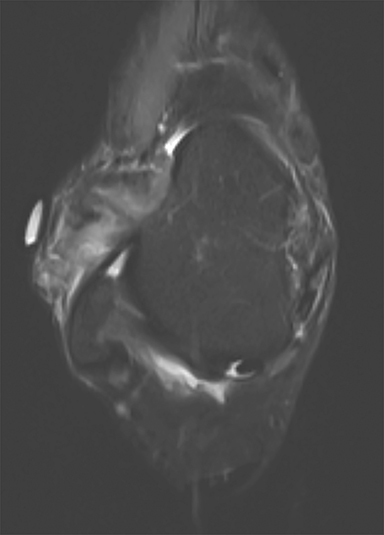
Synovial chondromatosis is a benign disorder that involves the formation of cartilaginous nodules in the synovium of joints, tendon sheaths, and lining of the adjacent extraarticular bursae.53 These nodules can become detached and exist as bodies in the joint space.53 Synovial chondromatosis occurs in large joints such as the hip, elbow, knee (Figure 5) and shoulder joint.54 The disorder can appear at any age, but is most prevalent in the 4th and 5th decades of life.54 Males are more affected than females in a ratio of 2-4:1.55,56 The appearance of synovial chondromatosis on MRI depends on the presence of synovial proliferation, formation of loose bodies, and degree of mineralization.57 Synovial chondromatosis is termed synovial osteochondromatosis if the intraarticular bodies are mineralized.55
Ganglion cysts
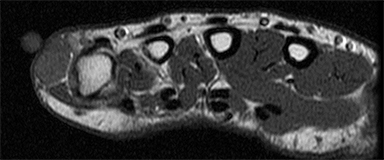
Ganglion cysts are benign cystic masses that usually appear as unilocular or multilocular fluid signal masses adjacent to a joint or tendon sheath.58 Women are approximately three times more likely to be affected than men.59 Ganglion cysts are most common on the dorsal surface of the wrist. Ganglion cysts may affect people of all ages, but more commonly affect individuals between the ages of 25 and 45.60 Very small cysts may mimic a small effusion, but the scarcity of fluid in the joint and the focal nature of the fluid should provide clues for diagnosis.61 The cysts typically have low signal intensity on T1-weighted images, but may appear hyperintense if they have high proteinaceous content or hemorrhage.62 Ganglion cysts typically have high signal on T2-weighted sequences62 and show peripheral enhancement after administration of intravenous contrast.61 Ruptured ganglion cysts are often irregularly delineated with pericapsular edema on T2-weighted sequences.61
Angioleiomyomas
Angioleiomyomas are benign smooth muscle tumors with prominent vascular components.63 Angioleiomyomas are more common in women.64 The peak incidence occurs between the 3rd and 6th decades of life.64 Angioleiomyomas can arise anywhere in the body, with a predilection for the lower extremities, and are painful in 60% of cases.64 On T2-weighted MRI, the lesions exhibit mixed areas of hyperintense and isointense signal.65-67 The T2-hyperintense areas correspond to smooth muscle bundle cells and show strong enhancement with contrast.65-67 The isointense areas correspond to fibrous tissue or intravascular thrombi.65-67
Intravascular papillary endothelial hyperplasia (IPEH)/Masson’s tumor
Intravascular papillary endothelial hyperplasia is a benign vascular disease characterized by a proliferation of the endothelium in blood vessels.68 It is most common in young adults, and women are more often affected than men.69 It occurs principally in the skin and subcutaneous soft tissues of fingers, trunk, head and neck region, and rarely in the oral region, appearing as a small superficial red to blue mass.70 T1-weighted sequences can display homogeneous signal (Figure 6A).71 On T2-weighted images, the lesions exhibit a heterogeneous hyperintense signal with foci of low intensity signal (Figure 6B)71 and have variable enhancement after administration of intravenous contrast (Figure 6C).
MRI: Malignant entities confused with synovial sarcoma
Lymphoma
Low-grade lymphomas such as follicular lymphoma may be mistaken for SS. Follicular lymphoma accounts for about 45% of all non-Hodgkin’s lymphoma.72 These are low-grade lymphomas, but have the propensity to transform to a more aggressive lymphoma.72 Lymphomas are usually T1 isointense or hypointense (Figure 7A), T2 hyperintense (Figure 7B), and demonstrate enhancement after administration of intravenous contrast (Figure 7C).
Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytomas)
The etiology and origin of UPS are unclear.73 This tumor occurs mostly in middle to late adulthood and occurs in the proximal region of the lower extremities.74 Undifferentiated pleomorphic sarcoma is usually low to intermediate signal intensity on T1-weighted sequences and intermediate to high on T2-weighted sequences.75 Myxoid UPS tends to be hyperintense on T2-weighted sequences due to the high water content of its lesions.75
Extraskeletal myxoid chondrosarcoma
Extraskeletal myxoid chondrosarcoma (EMC) is a rare subtype of chondrosarcoma.76 The median age of diagnosis is approximately 50 years of age, with 50% of patients between the ages of 41 and 60 years.77 Males are more commonly affected than females; the ratio is 2:1.78 There is a predilection for involvement of the lower (62%) and upper extremities (17%).77,78 EMC has a characteristic chromosomal translocation, typically t(9;22)(q22;q12.2), fusing EWSR1 to NR4A3, and the EWSR1 rearrangement can be detected by fluorescent in situ hybridization.79 The chromosomal translocation results in a fusion gene product responsible for alterations in cell growth and differentiation.79 This malignant neoplasm is best evaluated by MRI, which allows for visualization of myxoid stroma and the multinodular and well-defined borders of the mass.80 EMC tends to be isointense or hypointense on T1-weighted imaging and T2 hyperintense on T2-weighted imaging.79
Liposarcoma
Liposarcomas originate from primitive mesenchymal cells rather than mature fat cells.81 Therefore, the presence of adipocytes is not necessary for the development of this common soft-tissue tumor.81 Liposarcoma is primarily an adult tumor, with a peak incidence between the 4th and 6th decades of life.81 Males are slightly more often affected than females.81 Liposarcomas most commonly occcur in the thigh and the retroperitoneum.81 The myxoid histology is the most common feature of liposarcoma, making up 45-55% of all lesions.81 Myxoid liposarcomas lesions have low/intermediate T1 signal intensity (Figure 8A) due to the myxoid stroma and high water content of the myxomatous elements and very high T2 signal intensity (Figure 8B).82 The presence and relative proportion of round cells may decrease the water content of the tumor, resulting in low to intermediate T2 signal.83, 84 Most lesions demonstrate intense enhancement after administration of intravenous contrast. Encapsulated margins and thick internal septations are often visible.82
Epithelioid sarcomas
Epithelioid sarcomas are malignant tumors with unknown histogenesis.85 It is the most common soft tissue sarcoma in the hand and wrist.85 It occurs principally in the fingers, hands and forearms, but it can also occur in other sites, including in the musculature.86 Regional lymphadenopathy is often present, as this tumor has a propensity to spread to the lymph nodes.86 This tumor is prevalent in adolescents and young adults between 10 and 35 years of age.85 Males are affected twice as often as females.85 This tumor has variable MRI signal characteristics and should be suspected in patients with or without locally enlarged lymph nodes, as well as in patients with ulcerating cutaneous nodes.86
Melanoma
Melanomas are tumors that arise from melanocytes.87 Poorer prognosis is associated with a younger age of diagnosis, family history, and personal history of melanoma.87
Melanomas tend to be isointense or hyperintense on T2-weighted images (Figure 9), and are often hyperintense on T1-weighted sequences.88 The presence of melanin and blood products within melanomas tend to make these tumors hyperintense on T1-weighted sequences.
Conclusion
Accurate diagnosis of synovial sarcoma can impact prognosis and overall survival rates by maximizing the chances of appropriate and aggressive treatment. Although synovial sarcomas are most prevalent in children and young adults, and tend to occur in the extremities and can occur at any age and in any location in the body. We recommend all imaging studies be reviewed by a radiologist with experience in evaluating musculoskeletal tumors. A lesion should be considered a potential synovial sarcoma if it has any suspicious features. The authors recommend biopsy of all suspicious lesions and use of FISH for confirmation of the diagnosis of synovial sarcoma.
References
- Goldblum JR, Flope AL, Weiss SW. Malignant soft tissue tumors of uncertain type. In: Enzinger & Weiss’s Soft Tissue Tumors. Philadelphia: Elsevier; 2013:1052–1070.
- Milchgrub S, Ghandur-Mnaymneh L, Dorfman HD, et al. Synovial sarcoma with extensive osteoid and bone formation. Am J Surg Pathol. 1993;17(4):357-363.
- Miettinen M, Virtanen I. Synovial sarcoma--a misnomer. Am J Pathol. 1984;117(1):18-25.
- Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res. 2004;(419):155-161.
- Cadman NL, Soule EH, Kelly PJ. Synovial sarcoma; An analysis of 34 tumors. Cancer. 1965;18:613-627.
- Kransdorf MJ. Malignant soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164(1):129-134.
- Smith ME, Fisher C, Wilkinson LS, et al. Synovial sarcoma lack synovial differentiation. Histopathology. 1995;26(3):279-281.
- Haldar M, Hancock JD, Coffin CM, et al. A conditional mouse model of synovial sarcoma: insights into a myogenic origin. Cancer Cell. 2007;11(4):375-388.
- Skytting B. Synovial sarcoma. A Scandinavian sarcoma group project. Acta Orthop Scand Suppl. 2000;291:1-28.
- Sistla R, Tameem A, Vidyasagar JVS. Intra articular synovial sarcoma. Indian J Pathol Microbiol. 2010;53(1):115-116.
- Enzinger, FM, Weiss SW. Synovial sarcoma. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:757-786.
- Bakri A, Shinagare AB, Krajewski KM, et al. Synovial sarcoma: imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. AJR Am J Roentgenol. 2012;199(2):W208-215.
- Krieg AH, Hefti F, Speth BM, et al. Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011;22(2):458-467.
- Enzinger, FM, Weiss SW. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby, 1995:39
- Mansuy L, Bernier V, Ranchère-Vince D, et al. Synovial sarcoma in children and adolescents. Bull Cancer. 2016;103(2):210-218.
- Wilkerson BW, Crim JR, Hung M, et al. Characterization of synovial sarcoma calcification. AJR Am J Roentgenol. 2012;199(6):W730-734.
- Jones BC, Sundaram M, Kransdorf MJ. Synovial sarcoma: MR imaging findings in 34 patients. AJR Am J Roentgenol. 1993;161(4):827-830.
- Hasegawa T, Yokoyama R, Matsuno Y, et al. Prognostic significance of histologic grade and nuclear expression of beta-catenin in synovial sarcoma. Hum Pathol. 2001;32(3):257-263.
- Frazier AA, Franks TJ, Pugatch RD, et al. From the archives of the AFIP: Pleuropulmonary synovial sarcoma. Radiographics. 2006;26(3):923-940.
- Bixby SD, Hettmer S, Taylor GA, et al. Synovial sarcoma in children: imaging features and common benign mimics. AJR Am J Roentgenol. 2010;195(4):1026-1032.
- Chee DWY, Peh WCG, Shek TWH. Pictorial essay: imaging of peripheral nerve sheath tumours. Can Assoc Radiol J. 2011;62(3):176-182.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52(3):229-239.
- Enzinger, FM, Weiss SW. Benign tumors of peripheral nerves. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:844.
- Kakkar C, Shetty CM, Koteshwara P, et al. Telltale signs of peripheral neurogenic tumors on magnetic resonance imaging. Indian J Radiol Imaging. 2015;25(4):453-458.
- Anil G, Tan TY. CT and MRI evaluation of nerve sheath tumors of the cervical vagus nerve. AJR Am J Roentgenol. 2011;197(1):195-201.
- Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194(6):1568-1574.
- Enzinger, FM, Weiss SW. Benign tumors and tumorlike lesions of blood vessels. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:579
- Enzinger, FM, Weiss SW. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:581
- Marchuk DA. Pathogenesis of hemangioma. J Clin Invest. 2001;107(6):665-666.
- Cross JJ, Antoun NM, Laing RJ, et al. Imaging of compressive vertebral haemangiomas. Eur Radiol. 2000;10(6):997-1002.
- Ross JS, Masaryk TJ, Modic MT, et al. Vertebral hemangiomas: MR imaging. Radiology. 1987;165(1):165-169.
- Navarro OM. Magnetic resonance imaging of pediatric soft-tissue vascular anomalies. Pediatr Radiol. 2016;46(6):891-901.
- Sheldon PJ, Forrester DM, Learch TJ. Imaging of intraarticular masses. Radiographics. 2005;25(1):105-119.
- Enzinger, FM, Weiss SW. Benign lipomatous tumors. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:394
- Bolen JW, Thorning D. Spindle-cell lipoma. A clinical, light- and electron-microscopical study. Am J Surg Pathol. 1981;5(5):435-441.
- Enzinger FM. Benign lipomatous tumors simulating a sarcoma: In Enzinger FM. Management of primary bone and soft tissue tumors. Chicago: Year Book Medical Publishers; 1977: 11.
- Gorelkin L, Conrad-England R. Spindle cell lipoma: a benign lipoma variant with potential hazards of diagnostic misinterpretation. South Med J. 1978;71(9):1163-1164.
- McDaniel RK, Newland JR, Chiles DG. Intraoral spindle cell lipoma: case report with correlated light and electron microscopy. Oral Surg Oral Med Oral Pathol. 1984;57(1):52-57.
- Meister P. Spindle cell lipoma (report of 2 cases and differential diagnosis). Beitr Pathol. 1977;161(4):376-384.
- Bancroft LW, Kransdorf MJ, Peterson JJ, et al. Imaging characteristics of spindle cell lipoma. AJR Am J Roentgenol. 2003;181(5):1251-1254.
- Frassica FJ, Bhimani MA, McCarthy EF, et al. Pigmented villonodular synovitis of the hip and knee. Am Fam Physician. 1999;60(5):1404-1410; discussion 1415.
- Murphey MD, Rhee JH, Lewis RB, et al. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics. 2008;28(5):1493-1518.
- Lynskey SJ, Pianta MJ. MRI and thallium features of pigmented villonodular synovitis and giant cell tumours of tendon sheaths: a retrospective single centre study of imaging and literature review. Br J Radiol. 2015;88(1056):20150528.
- Xie G, Jiang N, Liang C, et al. Pigmented villonodular synovitis: a retrospective multicenter study of 237 cases. PLoS ONE. 2015;10(3):e0121451.
- Murphey MD, Rhee JH, Lewis RB, et al. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics. 2008;28(5):1493-1518.
- Narváez JA, Narváez J, Ortega R, et al. Hypointense synovial lesions on T2-weighted images: differential diagnosis with pathologic correlation. AJR Am J Roentgenol. 2003;181(3):761-769.
- Nair AV, Nazar P, Sekhar R, et al. Morel-Lavallée lesion: A closed degloving injury that requires real attention. The Indian Journal of Radiology & Imaging. 2014;24(3):288-290.
- Kottmeier SA, Wilson SC, Born CT, et al. Surgical management of soft tissue lesions associated with pelvic ring injury. Clin Orthop Relat Res. 1996;(329):46-53.
- Kudsk KA, Sheldon GF, Walton RL. Degloving injuries of the extremities and torso. J Trauma. 1981;21(10):835-839.
- Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellée lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
- Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
- Mellado JM, Pérez del Palomar L, Díaz L, et al. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
- Enzinger, FM, Weiss SW. Cartilaginous soft tissue tumors. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:997
- Kiritsi O, Tsitas K, Grollios G. A case of idiopathic bursal synovial chondromatosis resembling rheumatoid arthritis. Hippokratia. 2009;13(1):
- 61-63.
- Murphey MD, Vidal JA, Fanburg-Smith JC, DA Gajewski. Imaging of Synovial Chondromatosis with Radiologic-Pathologic Correlation. Radiographics. 2007;27(5):1465–1488.
- Narváez JA, Narváez J, Ortega R, et al. Hypointense synovial lesions on T2-weighted images: differential diagnosis with pathologic correlation. AJR Am J Roentgenol. 2003;181(3):761-769.
- Murphey MD, Vidal JA, Fanburg-Smith JC, et al. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
- Enzinger, FM, Weiss SW. Benign soft tissue tumors of uncertain type. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:1053
- Thornburg LE. Ganglions of the hand and wrist. J Am Acad Orthop Surg. 1999;7(4):231-238.
- Enzinger, FM, Weiss SW. Benign soft tissue tumors of uncertain type. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:1053
- Vanhoenacker FM, Eyselbergs M, Van Hul E, et al. Pseudotumoural soft tissue lesions of the hand and wrist: a pictorial review. Insights Imaging. 2011;2(3):319-333.
- Freire V, Guérini H, Campagna R, et al. Imaging of hand and wrist cysts: a clinical approach. AJR Am J Roentgenol. 2012;199(5):W618-628.
- Sun L, Zhu Y, Wang H. Angioleiomyoma, a rare intracranial tumor: 3 case report and a literature review. World J Surg Oncol. 2014;12:216.
- Ramesh P, Annapureddy SR, Khan F, et al. Angioleiomyoma: a clinical, pathological and radiological review. Int J Clin Pract. 2004;58(6):587-591.
- Rhatigan RM, Kim ZE. Leiomyoma arising adjacent to a maxillary tooth socket: an intraosseous leiomyoma presenting as an odotogenic lesion. South Med J 1976; 69 (4): 493-494.
- Goldblatt LI, Edesess RB. Central leiomyoma of the mandible. Oral Surg Oral Med Oral Pathol. 1977; 43 (4): 591–597.
- McMillan MD, Ferguson JW, Kardos TB. Mandibular vascular leiomyoma. Oral Surg Oral Med Pathol. 1985; 62 (4): 427–33.
- Tarallo M, Spagnoli AM, Fino P, et al. Masson’s tumor: a soft tissue tumor simulating a tendon cyst: case report. G Chir. 2012;33(1-2):34-37.
- Clearkin KP, Enzinger FM. Intravascular papillary endothelial hyperplasia. Arch Pathol Lab Med. 1976:100(8):441-444.
- Mahapatra QS, Sahai K, Malik A, et al. Intravascular papillary endothelial hyperplasia: An unusual histopathological entity. Indian Dermatol Online J. 2015;6(4):277-279.
- Liné A, Sanchez J, Jayyosi L, et al. Papillary endothelial hyperplasia (Masson’s tumor) in children. Ann Chir Plast Esthet. June 2016. doi:10.1016/j.anplas.2016.05.010.
- Mendenhall N, Lynch JW, Jr. The low-grade lymphomas. Semin Radiat Oncol. 1995;5:254-266.
- Matushansky I, Charytonowicz E, Mills J, et al. MFH classification: differentiating undifferentiated pleomorphic sarcoma in the 21st century. Expert Rev Anticancer Ther. 2009;9(8):1135-1144.
- Enjoji M, Hashimoto H, Tsuneyoshi M, et al. Malignant fibrous histiocytoma. A clinicopathologic study of 130 cases. Acta Pathol Jpn. 1980;30(5):727-741.
- Murphey MD, Gross TM, Rosenthal HG. From the archives of the AFIP. Musculoskeletal malignant fibrous histiocytoma: radiologic-pathologic correlation. Radiographics. 1994;14(4):807-826; quiz 827-828.
- Stacchiotti S, Dagrada GP, Morosi C, et al. Extraskeletal myxoid chondrosarcoma: tumor response to sunitinib. Clin Sarcoma Res. 2012;2(1):22.
- Drilon AD, Popat S, Bhuchar G, et al. Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer. 2008 15;113(12):3364-3371.
- Enzinger, FM, Weiss SW. Cartilaginous soft tissue tumors. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:998.
- Shao R, Lao IW, Wang L, et al. Clinicopathologic and radiologic features of extraskeletal myxoid chondrosarcoma: a retrospective study of 40 Chinese cases with literature review. Ann Diagn Pathol. 2016;23:14-20. doi: 10.1016/j.anndiagpath.2016.04.004. Epub 2016 Apr 13.
- Antonescu CR, Argani P, Erlandson RA, et al. Skeletal and extraskeletal myxoid chondrosarcoma: a comparative clinicopathologic, ultrastructural, and molecular study. Cancer. 1998;83(8):1504-1521.
- Enzinger, FM, Weiss SW. Liposarcoma. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:431-443
- Wortman JR, Tirumani SH, Jagannathan JP, et al. Primary Extremity Liposarcoma: MRI Features, Histopathology, and Clinical Outcomes. J Comput Assist Tomogr. 2016. doi:10.1097/RCT.0000000000000431.
- Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25(5):1371-1395.
- Rizer M, Singer AD, Edgar M, et al. The histological variants of liposarcoma: predictive MRI findings with prognostic implications, management, follow-up, and differential diagnosis. Skeletal Radiol. 2016;45(9):1193-1204.
- Enzinger, FM, Weiss SW. Malignant soft tissue tumors of uncertain type. In: Enzinger, FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis: Mosby; 1995:1074-1075
- Hanna SL, Kaste S, Jenkins JJ, et al. Epithelioid sarcoma: clinical, MR imaging and pathologic findings. Skeletal Radiol. 2002;31(7):400-412.
- Watts CG, Madronio C, Morton RL, et al. Clinical features associated with individuals at higher risk of melanoma: A population-based study. JAMA Dermatol. 2016 9. doi: 10.1001/jamadermatol.2016.3327.
- Gaviani P, Mullins ME, Braga TA, et al. Improved detection of metastatic melanoma by T2*-weighted imaging. AJNR Am J Neuroradiol. 2006;27(3):605-608.
Citation
Z W, R S. Distinguishing synovial sarcoma from benign and malignant mimics: MR Imaging indicators. Appl Radiol. 2018;(6):15-21.
June 7, 2018