Electronic-Cigarette or Vaping Associated Lung Injury (EVALI)
Images
CASE SUMMARY
A 37-year-old presented to the hospital with 3 days’ duration of increasing shortness of breath, subjective fevers, headache, dry cough, pleuritic chest pain, chest tightness, nausea, and vomiting. The patient denied any sick contacts and reported quitting cigarette smoking 4 years previously with an approximately 20-year-pack history. The patient reported currently vaping nicotine daily, along with occasional cocaine and marijuana use, reportedly last using intranasal cocaine 2 nights prior to presentation. The patient reported using a vaping device with nicotine juice, had last changed the heating coil about 1 week prior to presentation, and reported vaping about 3 pods a day. The patient was dyspneic, agitated, and had mild diffuse crackles on lung exam. Oxygen saturation was 79% on room air; Respiratory Virus Panel PCR, 4th generation HIV testing, Histoplasma antigen, and urinary antigens for streptococcus and legionella were all negative. Bronchoalveolar lavage was unremarkable. Bronchial biopsy was notable for fibrin exudates within airspaces, with occasional lipid-laden macrophages highlighted by Oil Red O stain. Laboratory values were notable for elevated d-dimer, procalcitonin, C-reactive protein, and leukocytes. A urine drug screen was positive for cannabis and cocaine.
IMAGING FINDINGS
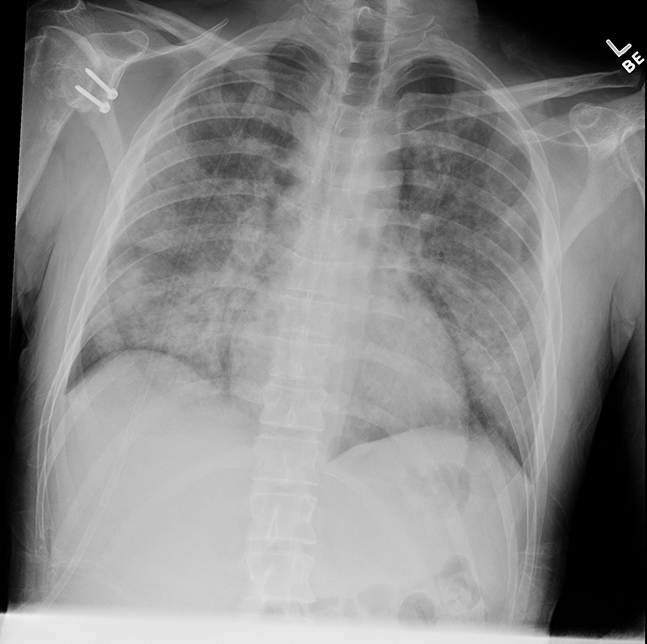
The patient’s chest X-ray (demonstrated diffuse bilateral airspace opacities without evidence of focal consolidation, masses, or pleural effusion. Radiologically, severe multifocal pneumonia and/or acute respiratory distress syndrome (ARDS) were considered along with Electronic cigarette or Vaping Associated Lung Injury (EVALI, Figure 1).
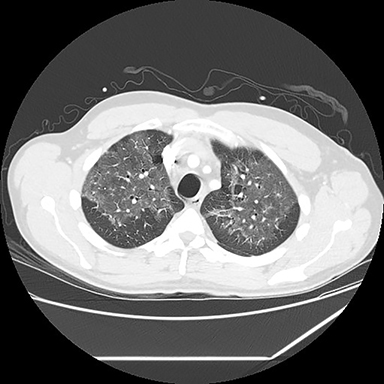
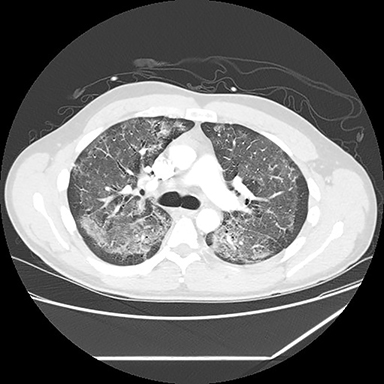
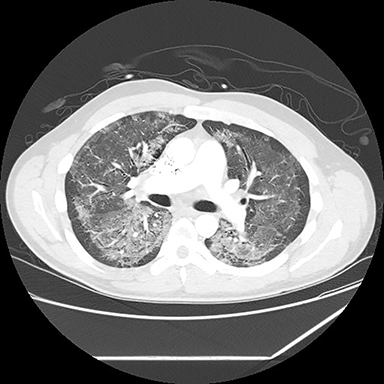
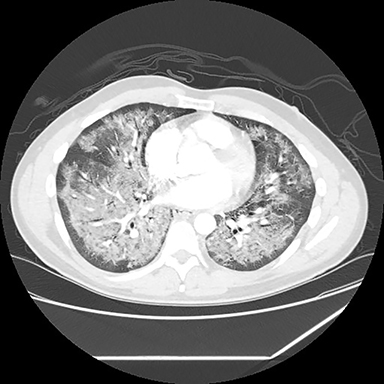
A computed tomography (CT) scan showed diffuse ground glass opacities, slightly more prominent in the lower lungs, which is consistent with EVALI in this clinical setting (Figure 2). The differential diagnosis for diffuse ground glass opacities is broad; infection, chronic interstitial diseases, vasculitis, drug toxicity, and other etiologies were considered. CT angiography of the chest showed no pulmonary embolism.
DIAGNOSIS
Electronic cigarette or Vaping Associated Lung Injury. The differential diagnosis included severe multifocal pneumonia, ARDS, diffuse alveolar hemorrhage, lipoid pneumonia, vasculitis, histoplasmosis, Pneumocystis jirovecii pneumonia, and cocaine-related lung injury.
DISCUSSION
As of February 4, 2020, the Centers for Disease Control and Prevention (CDC) reported 2,758 cases of EVALI, including 64 deaths.1 The majority of patients reported vaping both nicotine and marijuana products, raising the possibility of in vivo reactions forming novel compounds responsible for the lung injury;2 however, some patients reported using only nicotine or marijuana.3 The CDC further reported that most of the cases seemed to involve THC-containing products obtained from unofficial sources.4 On Nov. 8, 2019, the CDC reported Vitamin E as a potential causal agent based on bronchoalveolar lavage samples from 20 patients submitted to the CDC.1
The exact pathophysiology of EVALI is not known and may represent a spectrum of disease rather than a distinct entity. Butt et al reviewed lung biopsies from cases of confirmed or suspected EVALI, finding that all cases demonstrated histopathological findings of acute lung injury, including diffuse alveolar hemorrhage, organizing pneumonia, and/or acute fibrinous pneumonitis. While the researchers found no histological findings specific to EVALI, they identified foamy macrophages and pneumocyte vacuolization in all cases. The authors concluded that EVALI represents a form of chemical pneumonitis from a yet-to-be identified group of toxic substances.5
A case series of 53 patients in Illinois and Wisconsin described the presentation of EVALI with the relative frequency of symptoms as follows: constitutional symptoms (100%), respiratory symptoms (98%), and gastrointestinal symptoms (81%). They defined the patients as persons with e-cigarette use within 90 days prior to symptom onset and with bilateral infiltrates on chest imaging and no other explanatory causes for their symptoms. The patients’ median age was 19, 83% were male, 94% were hospitalized, 32% required intubation and mechanical ventilation, and 1 patient died.3
The initial clinical presentation of EVALI is nonspecific, but bilateral infiltrates on chest imaging permits substantially narrowing the differential diagnosis potentially suggesting this entity. Radiologically, the differential diagnosis is broad and includes pneumonia, ARDS, histoplasmosis, vasculitis, acute eosinophilic pneumonia, diffuse alveolar hemorrhage, bronchiolitis with interstitial lung disease, lipoid pneumonia, giant cell pneumonitis, HIV with alveolar infiltrate, and hypersensitivity pneumonitis. Complicating radiologic diagnosis, a number of poorly understood pulmonary complications and diseases, such as hypersensitivity pneumonitis, acute lung injury/diffuse alveolar hemorrhage, acute eosinophilic pneumonia, lipoid pneumonia, giant cell interstitial pneumonia, and respiratory-associated bronchiolitis-associated interstitial lung disease, have been attributed to, or may occur with, EVALI.7
While pulmonary infiltrates are widely considered inclusion criteria,3 no formal diagnostic imaging criteria exist for EVALI.4 However, radiologists play an important role in recognizing findings consistent with EVALI and may alert clinicians to initiate appropriate workup and treatment.7
An optimal treatment regimen has yet to be determined, but the CDC recently published treatment guidelines for EVALI.4 Acute treatments include corticosteroids, antibiotics for community-acquired pneumonia, and antivirals for influenza. Follow-up considerations include pulse oximetry, spirometry and diffusion capacity testing, followed by chest radiography, typically 1-2 weeks after initial insult. To prevent recurrence, the CDC advises avoiding the use of vaping devices with any nicotine or THC-containing juices, as both have been linked to EVALI.4
Further work is needed to establish formal clinical diagnostic criteria and to comprehensively and quantitatively catalog the spectrum of pathological and radiological manifestations of EVALI.
CONCLUSION
We present a case of EVALI in a 37-year-old patient, who was treated with noninvasive oxygen, ceftriaxone and azithromycin, and high-dose intravenous methylprednisone. Chest X-rays were performed daily and improved through the patient’s stay. A rapid recovery was made, the patient was weaned off oxygen, and was discharged on hospital Day 4 with a prednisone taper and nicotine patches. Overall findings and clinical course were highly consistent with EVALI. At follow up three weeks after discharge, the patient had reduced but not eliminated vaping, and denied any further symptoms.
As a relatively new entity with poorly understood pathophysiology and no formal diagnostic criteria, EVALI may not always be considered initially in patients presenting to the emergency department. While the full extent of radiological manifestations have yet to be determined, bilateral infiltrates on chest radiography and diffuse ground-glass opacities on CT may play a key role in properly diagnosing this entity and managing the increasing frequency of EVALI.
REFERENCES
- Outbreak of lung injury associated with e-cigarette use, or vaping. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Published 2019. Accessed February 24, 2020.
- Christiani D. Vaping-induced lung injury. NEJM New Engl J Med. 2019. doi:10.1056/nejme 1912032.
- Layden J, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin — Preliminary Report. NEJM New Engl J Med. 2019. doi:10.1056/nejmoa1911614.
- Siegel D, Jatlaoui T, Koumans E, et al. Update: Interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping product use-associated lung injury — United States, October 2019. Center for Disease Control and Prevention. https://www.cdc.gov/mmwr/volumes/68/wr/mm6841e3.htm. Published 2019. Accessed October 21, 2019.
- Butt Y, Smith M, Tazelaar H, et al. Pathology of vaping-associated lung injury. NEJM New Engl J Med. 2019. doi:10.1056/nejmc1913069.
- Henry T, Kanne J, Kligerman S. Imaging of vaping-associated lung disease. NEJM New Engl J Med. 2019;381(15):1486-1487. doi:10.1056/nejmc1911995.
Citation
WH K, C B, T S, P J, P M, J M, A P, D S. Electronic-Cigarette or Vaping Associated Lung Injury (EVALI). Appl Radiol. 2020;(2):42-44.
March 17, 2020