FDA clears FLUOROspeed XI RF table system from Shimadzu Medical Systems USA
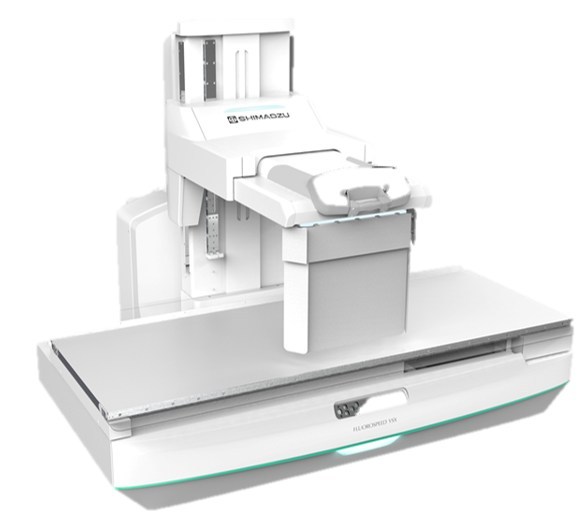 The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to Shimadzu Medical Systems USA for a new radiographic/fluoroscopic (RF) system, the FLUOROspeed X1. The newest U.S.-based product of Shimadzu’s FLUOROspeed series, the FLUOROspeed X1 is is a patient side conventional RF table system offering high image quality and numerous features to improve workflow and operator efficiencies, according to the Torrance, CA-headquartered company.
The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to Shimadzu Medical Systems USA for a new radiographic/fluoroscopic (RF) system, the FLUOROspeed X1. The newest U.S.-based product of Shimadzu’s FLUOROspeed series, the FLUOROspeed X1 is is a patient side conventional RF table system offering high image quality and numerous features to improve workflow and operator efficiencies, according to the Torrance, CA-headquartered company.
The RF system supports a 665 lb. static patient weight and 500 lb. all-motion weight. It comes equipped with a 17"x17" dynamic digital X-ray detector (FPD) in the table bucky. With its 31.5-inch aperture opening between table top and deck, the X1 provide access for imaging patients in wheelchairs and can also fit in smaller rooms with limited space. A second X-ray tube on an overhead rail can be added to increase system functionality and room versatility.
The X1 is designed for cost-effective general FR applications that include chest, abdomen, extremities, Upper GI's, modified swallows, and joint injections. An ambidextrous control handle for the imaging deck along with fingertip access to APR's, image recording functions and site-specific programmable function buttons, are all standard on the new X1 RF system