FDA Clears Technology Designed to Reduce Unnecessary Radiation During Fluoroscopy
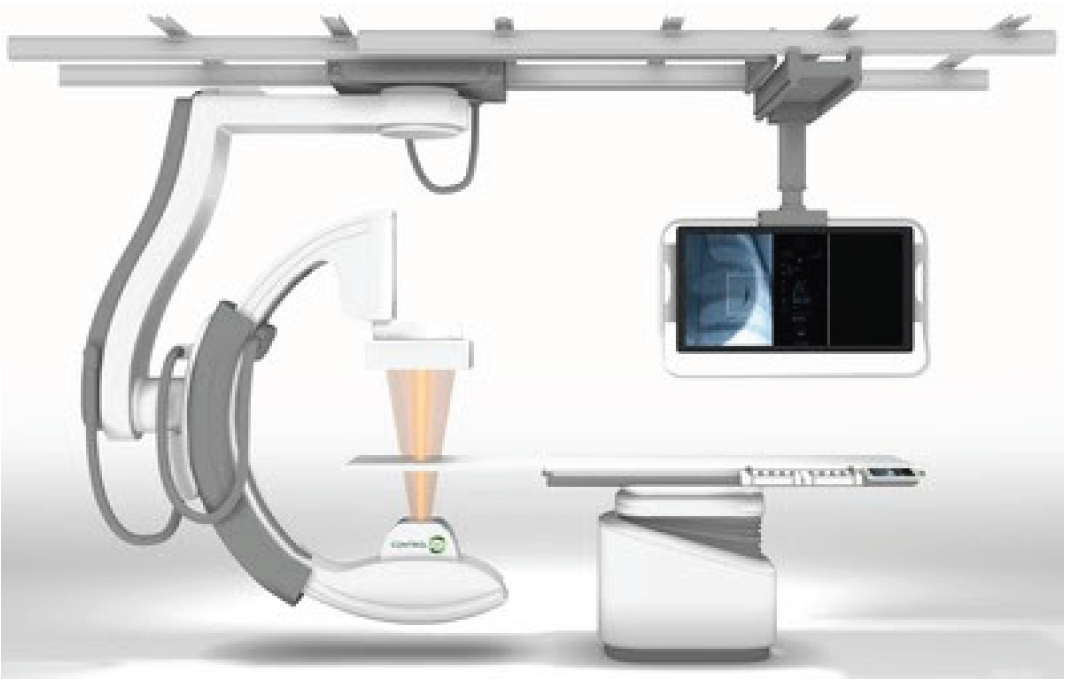 The US FDA has given 510(k) clearance to ControlRad, Inc. to market ControlRad Select, a technology that utilizes proprietary semi-transparent filters, a user-interface tablet, and image processing algorithms to reduce unnecessary radiation exposure during fluoroscopically guided procedures. The technology can be retrofitted onto customers' existing Siemens Artis zee interventional imaging systems. ControlRad also announced an exclusive agreement with Boston Scientific Corporation to sell the ControlRad Select technology.
The US FDA has given 510(k) clearance to ControlRad, Inc. to market ControlRad Select, a technology that utilizes proprietary semi-transparent filters, a user-interface tablet, and image processing algorithms to reduce unnecessary radiation exposure during fluoroscopically guided procedures. The technology can be retrofitted onto customers' existing Siemens Artis zee interventional imaging systems. ControlRad also announced an exclusive agreement with Boston Scientific Corporation to sell the ControlRad Select technology.
"The health risks to the medical staff due to lifetime radiation exposure in cath labs have been well documented, including increased incidence of cataracts, atherosclerosis and even left-brain tumors," stated Simon Dixon, MD, Chief of Cardiology at Beaumont Hospital, Royal Oak, Michigan. "We recently completed a clinical trial designed to evaluate how this novel technology might reduce radiation exposure in the cath lab. I have long been passionate about finding innovative ways to improve safety for my colleagues in the lab while they are performing lifesaving procedures for our patients."
"With the FDA clearance and the reach of the global medical device sales team, every cath, EP, and IR lab in the country that has a Siemens Artis zee will now have the opportunity to reduce their radiation dose by 85% without compromising image quality," stated Guillaume Bailliard, CEO of ControlRad.
