High attenuation in the lungs on CT: Beyond calcified granulomas
Images
High attenuation in the lungs is not infrequently encountered on computed tomography (CT). Not all significantly radiopaque lung findings are calcified. Iodine (a halogen) as well as a variety of metals besides calcium cause high attenuation. Pulmonary calcification may occur in two ways: dystrophic and metastatic. The former denotes calcium deposition in damaged tissue (caseation, necrosis, fibrosis) despite normocalcemia. Metastatic calcification is defined later.
High attenuation is recognizable on soft-tissue windows, but is easily missed if only lung windows are assessed. The finding may be striking on maximum intensity projection (MIP) images, if available. The literature offers assorted definitions for high attenuation; for example, visually opaque as bone or > 200 HU.1,2 No single definition applies in every instance. In practice, however, these criteria may be useful in equivocal cases.
Images processed with high-spatial-frequency reconstruction algorithms for lung analysis may cause the false appearance of high attenuation, such as in tiny pulmonary nodules. This predisposes to the premature conclusion of benignity. Therefore, we suggest that any suspicion of high attenuation be confirmed on standard algorithm images.
While in many cases the presence of high attenuation is ancillary and not required for diagnosis, it can be helpful in the imaging interpretation of some entities (eg, amiodarone). Mindful of the audience, we try to cover the gamut of high attenuation specifically within the lungs on CT, using intentional brevity and emphasizing features that lend themselves to a diagnosis.
Airway and vascular processes
An airway or vascular process is discernible on cross-sectional imaging, whether purely by localization or by the presence of a tubular or branching process. Airway processes may obstruct, causing any combination of mucus plugging, bronchial dilation or bronchiectasis, airway-centric opacities, atelectasis, consolidation, and air trapping. Decreased perfusion due to hypoxic vasoconstriction or due to increased pressures from air trapping is possible. In vascular processes, decreased perfusion suggests oligemia or intrinsic vascular pathology.
Airway
With respect to high attenuation alone, the presence of eccentric or stippled calcifications within an airway-associated lesion should trigger further investigation, as these patterns of calcification are indeterminate for malignancy. On the other hand, popcorn calcification is essentially diagnostic of a hamartoma and would preclude unnecessary workup.
A calcified or ossified endobronchial lesion without an associated soft-tissue component constitutes a broncholith, having likely reached its location by erosion of a calcified lymph node through an airway wall. The presence of calcified mediastinal/hilar lymph nodes elsewhere may implicate sequela of histoplasmosis or tuberculosis. Broncholithiasis does not have to be endobronchial. Its definition includes calcified peribronchial lymph nodes that cause adjacent airway distortion and/or obstruction.3 Less common causes of broncholithiasis include aspiration of foreign bodies that either are radiopaque or later calcify, and rarely endoluminal sequestration of calcified bronchial cartilage.4
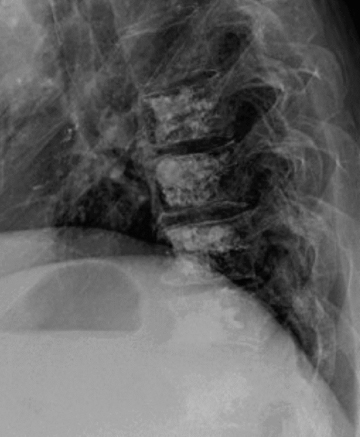
Ideally, distal airway and airway-centric high-attenuating opacities are demonstrated in the setting of oral contrast aspiration (Figure 1). Uniform high attenuation makes infection unlikely. When confluent, the diagnosis becomes challenging and relies more heavily on the sequence of events relative to oral contrast administration as well as on history (eg, CNS disorder). Ancillary findings of aspiration may include chemical pneumonitis and secondary pneumonia.5 Aspiration of elemental mercury is considered in the appropriate setting.
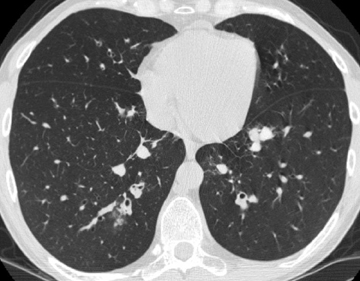
In asthmatics, moderate-to-severe central bronchiectasis affecting 3 or more lobes, bronchial wall thickening, mucoid impaction, and centrilobular lung nodules are suggestive of allergic bronchopulmonary aspergillosis (ABPA) (Figure 2).6 The same features are less useful in poorly controlled cystic fibrosis. High-attenuating mucus relative to normal skeletal muscle adds specificity to the diagnosis in asthmatics and may be the finding that elicits the diagnosis in cystic fibrosis. The higher density is attributed to inspissation of mucus and deposition of calcium salts and metals.7 Of note, persistent mucus plugging in general may calcify.
Extensive or diffuse tracheobronchial high attenuation (eg, warfarin therapy, relapsing polychondritis) will not be discussed.
Vascular
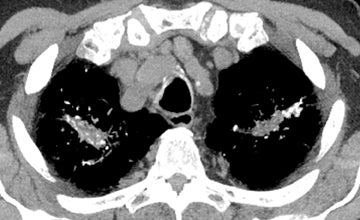
Atherosclerosis of the pulmonary circulation, characterized by vascular wall calcification, is primarily seen with pulmonary hypertension and congenital heart disease, but is not typically required for the diagnosis of either entity. Increased wall shear stress and high flow associated with elevated mean pulmonary arterial pressures probably contribute to its development.8
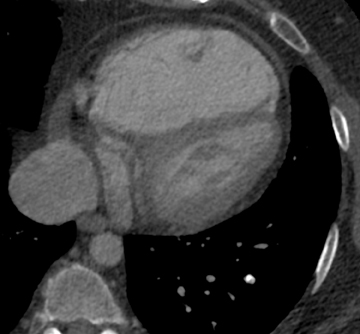
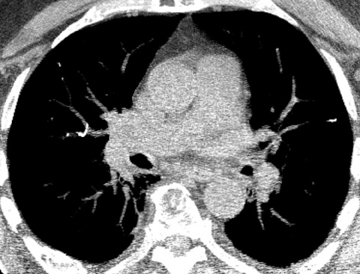
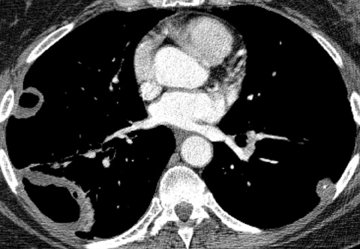
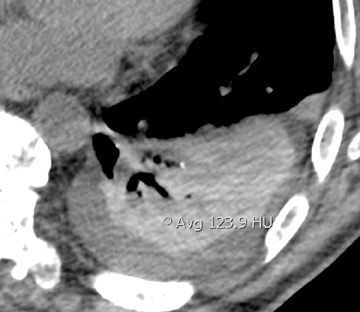
The majority of entities causing vascular high attenuation in the lungs are embolic in nature. Calcified thrombus is a known association of chronic thromboembolic disease (Figure 3), but is not integral to the diagnosis. Chronic thromboembolic disease is confirmed by identifying an appropriate filling defect pattern in the pulmonary arteries (eg, webs, bands, eccentric thrombus) with or without pulmonary hypertension, collateral systemic supply, parenchymal scarring from prior infarction, mosaic attenuation, and bronchial dilation.9 Occasionally, acute thromboembolic disease is detectable on unenhanced chest CT as high attenuating clot.10
Although rarely reported, predominantly intravascular calcifications have been described in the setting of fat embolism and ARDS, thought to be related to vascular injury and subsequent thrombosis.11
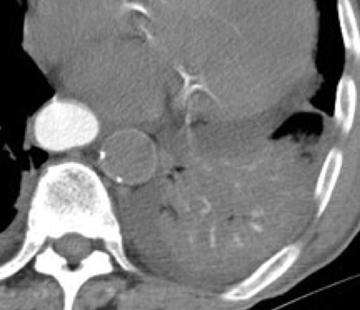
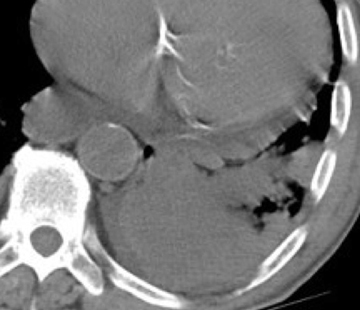
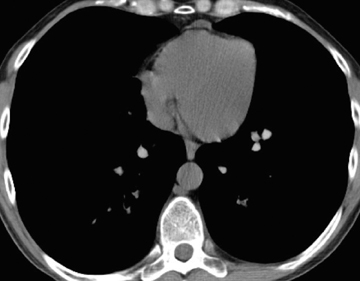
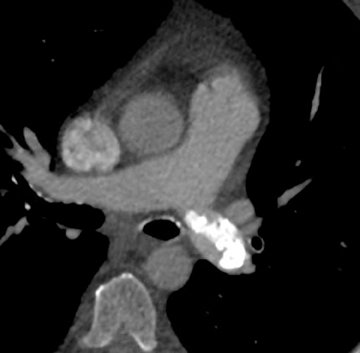
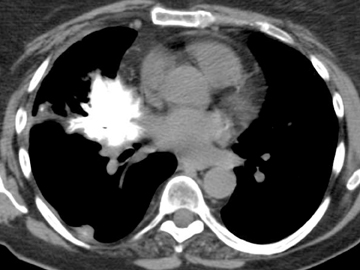
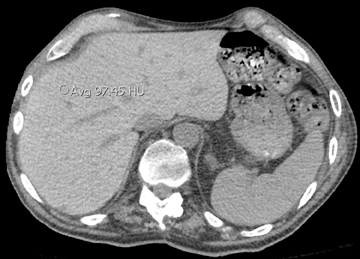
In cement embolism, the pulmonary arterial filling defects are purely high attenuating and tubular in configuration, occasionally straddling bifurcation points (Figure 4). The presence of post-vertebroplasty change with or without perivertebral venous leakage of acrylic cement is confirmatory. The caliber of the perivertebral veins is such that the pulmonary emboli are typically segmental or subsegmental in location.12
Upon contacting circulating blood through accidental or intentional administration, elemental mercury becomes spherical owing to interface tension. This is unlike iodinated contrast, which readily intermixes with blood. Mercury embolizes to dependent locations within lung due to its higher density than water. The droplets may coalesce and become tubular within vessels. The diagnosis is based on pure high-attenuating material in a generally symmetric and bilateral vascular distribution, arborized, if extensive, and supported by the presence of droplets in the right side of the heart or in other vascular beds. If fortuitous, images will show subcutaneous deposits in the injected extremity.13 History and clinical findings secure the diagnosis.
Other foreign body emboli (eg, glue from treatment of an arteriovenous malformation) may be diagnosed by clinical history and sequence of events. Central venous catheters occasionally break spontaneously and migrate into the lungs. The shearing of catheters by introducing needles is said to have been more common prior to the Seldinger technique.14
Ossification in pulmonary artery sarcomas may initially conjure the diagnosis of calcified chronic pulmonary thromboembolic disease, but the imaging appearances are dissimilar. The filling defects associated with sarcomas tend to span the entire lumen of proximal pulmonary arteries, unusual in chronic thromboembolic disease. Other suggestive features include luminal expansion, tissue invasion, and heterogeneous contrast enhancement. Finally, ossification in pulmonary artery sarcomas is more peripheral and nodular or stippled compared to chronic thromboembolic disease and is more often seen with osteo- or chondrosarcoma subtypes.15
Parenchymal processes
Solitary pulmonary nodule
The workup of a solitary pulmonary nodule has been well described in the literature, with diagnosis/management depending on nodule characteristics, stability, and risk factors for malignancy.16,17 With exceptions, diffuse, central, lamellar, and popcorn type calcifications are indicative of benign etiologies, while stippled and eccentric patterns are indeterminate. The most common causes of solitary pulmonary nodules are hamartomas, granulomas, and malignancy. As above, the diagnosis of a hamartoma (or even multiple hamartomas) is made simple by the infrequent occurrence of popcorn calcification.
Other lesions
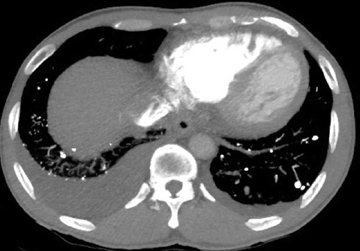
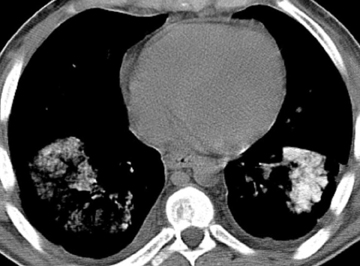
Calcified and ossified metastases are uncommon, but should be entertained if the calcification pattern is indeterminate or if certain primary malignancies are known. Osteosarcoma and chondrosarcoma occur more frequently. Less common are other sarcomas (eg, synovial sarcoma), mucinous adenocarcinomas (eg, breast, colon, ovary), and thyroid malignancies (eg, medullary carcinoma) (Figure 5). Lesions that newly calcify may represent treated metastases in the appropriate setting (eg, choriocarcinoma). Carney’s triad is rare and difficult to diagnose prospectively. The triad includes benign pulmonary chondroma (commonly calcified), gastrointestinal stromal tumor, and extra-adrenal paranganglioma.1,12
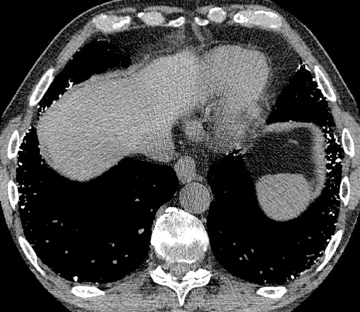
When considering calcified/ossified metastatic disease, the differential may include nodular amyloid, hyalinizing granulomas, and rheumatoid nodules (Figure 6). In practice, these are difficult to differentiate on CT, having in common well-marginated solitary or multiple pulmonary nodules/masses that can cavitate and calcify. In addition, imaging findings may precede or occur in the absence of clinical findings. For amyloid, chronic inflammatory diseases and multiple myeloma are predisposing conditions and may raise the possibility.18,19 Thin-walled air cysts also help distinguish amyloid but may be seen with metastases from the aforementioned primaries.20-22 Although Histoplasma and Mycobacterium are possible causative agents of hyalinizing granulomas, calcified lymph nodes have not been described in association.12,23 The bias of necrobiotic lung nodules for peripheral or pleural locations and their ascribed waxing and waning course are of limited diagnostic value, even with known rheumatoid arthritis and subcutaneous necrobiotic nodules.24
High attenuating lung nodules on the order of 2 to 5 mm are frequently labeled calcified granulomas. Doing so is not always accurate. It is appropriate in the presence of calcified mediastinal/hilar lymph nodes, which typically implicate disseminated histoplasmosis, less commonly miliary tuberculosis, as the culprit. Splenic and/or liver calcifications are more suggestive of the former.12,25
In the absence of calcified lymph nodes and splenic or liver calcifications, the late sequelae of a viral pneumonia, particularly varicella (chickenpox), is entertained. The calcified lung nodules are said to be on the order of 1-2 mm.25
Dystrophic calcifications occur in other infectious settings, but are less integral to the diagnosis. Examples include apical tuberculous fibronodular changes, symmetric bilateral ground glass opacities in pneumocystis jiroveci pneumonia, cavitary filling defects of aspergillomas, hepatic and pulmonary hydatid cysts, and pleuroparenchymal changes in paragonimiasis, all of which may calcify, rarely in some cases.1,2,8,25,26
Keep in mind that while calcification is often taken to mean healing, the underlying infection could still be active.1
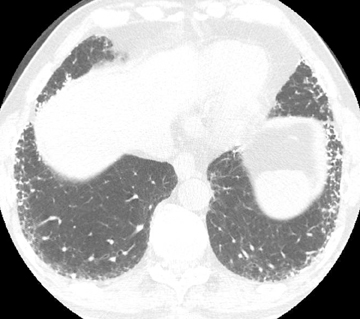
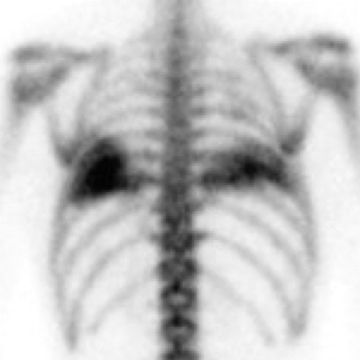
With evidence of chronic pulmonary venous hypertension, a distinctively lower-lung-predominant distribution of high-attenuating nodules (reported anywhere from 1-8 mm in size) is highly suggestive of diffuse pulmonary ossification, nodular type (Figure 7). The nodules may be misinterpreted as calcified granulomas. The classic example of diffuse pulmonary ossification is chronic mitral stenosis. In the case of rheumatic heart disease, findings often comprise a calcified mitral valve, an enlarged left atrium with or without atrial wall calcifications, and pulmonary hypertension.
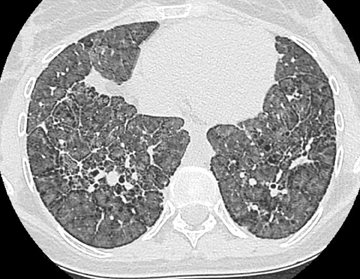
Nodular ossification also occurs in the setting of pulmonary fibrosis and may be similarly misconstrued as calcified granulomatous disease. Its occurrence only in areas of fibrosis aids in the diagnosis. Dendriform ossification, however, is more commonly described in this setting (Figure 8). This second type of diffuse pulmonary ossification reflects linear/branching high attenuation along the interstitium and is more reminiscent of bony trabeculation. Chronic or repeated lung injury is thought to be the substrate for diffuse pulmonary ossification. In fact, ossification may occur in a number of entities (eg, amyloid, tuberculosis). Suffice it to say that any longstanding calcification is transformable to bone.8,27-30
The differential for bilateral centrilobular ground glass lung opacities is considerably broad. If high attenuation is felt to be associated, however, then recurrent alveolar hemorrhage resulting in deposition of iron and variably of calcium is possible, whether from idiopathic or secondary pulmonary hemosiderosis (Figure 9). Support for a secondary cause may come from evidence of increased pulmonary capillary/venous pressures, collagen vascular disease, or hemorrhagic disease. Hemosiderosis from mitral stenosis tends to distribute in the lower lungs.12,29,31
Unilateral or bilateral 3- to 10-mm fluffy centrilobular lung nodules with variably perceptible high attenuation are a common manifestation of metastatic calcification (Figure 10) and may resemble hemosiderosis. These nodules can densely calcify or densely consolidate. The absence of upper-lung involvement does not exclude the diagnosis despite a predilection for more alkaline environments. Development requires a derangement in calcium or phosphate metabolism leading to hypercalcemia and subsequent calcium deposition in normal tissues. Knowledge of a predisposing benign or malignant condition aids in the diagnosis (eg, chronic renal failure, multiple myeloma). Vascular calcifications in the chest wall and uptake on bone scan are also supportive.12,32,33
Bilateral mid- to upper-lung predominant < 5 mm perilymphatic nodules with or without mass-like conglomeration in both upper lungs elicits a differential of inorganic dust exposure and sarcoidosis (Figure 11). Added calcification or ossification contributes little to the diagnosis, although it is exceedingly rare in sarcoid lung nodules.25 Silicosis and coal workers’ pneumoconiosis are largely indistinguishable despite slight differences in nodule margins and calcification pattern. Berylliosis and pulmonary sarcoidosis mimic one another on imaging and pathology and have more of a tendency for interstitial thickening. Unilateral mass-like consolidation should prompt consideration for malignancy.34 Egg-shell calcifications make berylliosis unlikely.
Silicoproteinosis is an acute form of silicosis. The alveolar proteinaceous filling that is characteristic of this process may manifest with nonspecific consolidation and nodular or patchy ground glass, but the diagnosis is perhaps most suggested by crazy paving associated with calcification in areas of consolidation.33
Baritosis (barium), stannosis (tin), and siderosis (iron) are radiopaque dust exposures that cause small lung nodules.1,12 These are very rare conditions and difficult to diagnose prospectively without a contributory history. Fibrotic changes in the lungs argue against pure radiopaque dust exposure, unlike in inorganic dusts, combined exposures (eg, silicosiderosis), and sarcoid.34
Diffuse parenchymal (or alveolar septal) amyloidosis is in the differential of perilymphatic nodularity but has no zonal predilection and is not associated with bilateral mass-like consolidation.12,35 As with nodular amyloidosis, thin-walled air cysts would support the diagnosis.36
Talcosis, whether inhalational or intravenous, is in the differential of bilateral mass-like consolidation but has no zonal predilection. The diagnosis may be considered if the consolidation is seen with a background of ground glass, diffuse centrilobular nodules (≤ 4 mm), or profuse micronodules (< 1 mm), all without zonal predominance. High attenuation, if perceptible within the masses, can be diffuse. The addition of lower-lung-predominant panlobular emphysma suggests methylphenidate (Ritalin) abuse associated with intravenous talcosis.37 Without bilateral conglomerate masses, the diagnosis of talcosis becomes more difficult but may be considered for diffuse bilateral small centrilobular or tree-in-bud lung nodules.38,39
Although inconsistently seen, a crazy-paving pattern predominating in the mid- to lower-lung zones with extensive high-attenuating “interstitial” opacities suggests pulmonary alveolar microlithiasis. A genetic mutation in the lung’s phosphate transporter results in accumulation of alveolar calcium-phosphate microliths that tend to align along the septa, giving the illusion of interlobular septal thickening. Unresolved microliths create the ground glass appearance. Larger concretions may produce a sandstorm appearance of calcified micronodules, and confluence leads to diffusely high-attenuating consolidation, often posteriorly and along heart borders. Subpleural cysts and apical bullae may be seen.40-42
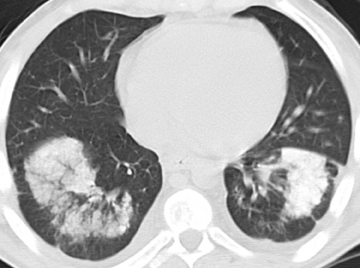
Amiodarone and iodinated oil embolism are in the differential of single or multiple high-attenuating areas of consolidation without associated lung nodules (Figure 12). Amiodarone is typically peripheral. The iodine moieties of the antiarrhythmic drug are responsible for the high attenuation, ranging from 82-174 HU according to one series. The presence of interstitial opacities or fibrosis would favor amiodarone, as would a high-attenuating liver or spleen (90-110 HU). Amiodarone toxicity versus simple lung accumulation cannot be differentiated on imaging alone.1,43 The diagnosis of iodinated oil embolism relies on knowledge of prior lymphangiography or transcatheter oil chemoembolization.12 In either case, history is integral to the diagnosis.
Suture material or staple lines are linear or curvilinear. They skirt the outer margins of the lung and may invaginate. Volume loss is invariably present due to partial lung resection. Fine detail of the material is not always appreciable using different windowing. Nonrecognition may cause confusion with one or more of the above entities, particularly if lung opacities are superimposed (eg, in the postoperative setting). On the other hand, these regions should be closely monitored for tumor recurrence in the appropriate setting.44
Finally, certain metallic opacities cause significant streak artifact and may require scout tomography and clinical history for elucidation (eg, bullets, fiducial markers).
Conclusion
High attenuation in the lungs is more than calcification. Acknowledging this adds complexity to its interpretation. At times, its presence is a welcome clue to otherwise nonspecific lung opacities. In many cases, however, the diagnosis will remain uncertain. Nevertheless, we hope this article leads to more confident interpretation.
References
- Chai JL, Patz EF Jr. CT of the lung: Patterns of calcification and other high-attenuation abnormalities. AJR Am J Roentgenol. 1994;162:1063-1066.
- Khan AN, Al-Jahdali HH, Allen CM, et al. The calcified lung nodule: What does it mean? Ann Thorac Med. 2010;5:67-79.
- Conces DJ Jr, Tarver RD, Vix VA. Broncholithiasis: CT features in 15 patients. AJR Am J Roentgenol. 1991;157:249-253.
- Seo JB, Song KS, Lee JS, et al. Broncholithiasis: Review of the causes with radiologic-pathologic correlation. Radiographics. 2002;22 Spec No:S199-213.
- Franquet T, Giménez A, Rosón N, et al. Aspiration diseases: Findings, pitfalls, and differential diagnosis. Radiographics. 2000;20:673-685.
- Ward S, Heyneman L, Lee MJ, et al. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. AJR Am J Roentgenol. 1999;173:937-942.
- Agarwal R. High attenuation mucoid impaction in allergic bronchopulmonary aspergillosis. World J Radiol. 2010;2:41-43.
- Chan ED, Morales DV, Welsh CH, et al. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 2002;165:1654-1669.
- Castañer E, Gallardo X, Ballesteros E, et al. CT diagnosis of chronic pulmonary thromboembolism. Radiographics. 2009;29:31-50.
- Kanne JP, Gotway MB, Thoongsuwan N, et al. Six cases of acute central pulmonary embolism revealed on unenhanced multidetector CT of the chest. AJR Am J Roentgenol. 2003;180:1661-1664.
- Hamrick-Turner J, Abbitt PL, Harrison RB, et al. Diffuse lung calcifications following fat emboli and adult respiratory distress syndromes: CT findings. J Thorac Imaging. 1994;9:47-50.
- Marchiori E, Souza AS Jr, Franquet T, et al. Diffuse high-attenuation pulmonary abnormalities: A pattern-oriented diagnostic approach on high-resolution CT. AJR Am J Roentgenol. 2005;184:273-282.
- Sichletidis L, Moustakas I, Chloros D, et al. Scattered micronodular high density lung opacities due to mercury embolism. Eur Radiol. 2004;14:2146-2147.
- Rossi SE, Goodman PC, Franquet T. Nonthrombotic pulmonary emboli. AJR Am J Roentgenol. 2000;174:1499-1508.
- Yi CA, Lee KS, Choe YH, et al. Computed tomography in pulmonary artery sarcoma: Distinguishing features from pulmonary embolic disease. J Comput Assist Tomogr. 2004;28:34-39.
- Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49.
- Tan BB, Flaherty KR, Kazerooni EA, et al. The solitary pulmonary nodule. Chest. 2003;123(Suppl):89S-96S.
- Slanetz PJ, Whitman GJ, Shepard JA, et al. Nodular pulmonary amyloidosis. AJR Am J Roentgenol. 1994;163:296.
- Georgiades CS, Neyman EG, Barish MA, et al. Amyloidosis: Review and CT manifestations. Radiographics. 2004;24:405-416.
- Kobayashi H, Matsuoka R, Kitamura S, et al. Sjögren’s syndrome with multiple bullae and pulmonary nodular amyloidosis. Chest. 1988;94:438-440.
- Jeong YJ, Lee KS, Chung MP, et al. Amyloidosis and lymphoproliferative disease in Sjögren syndrome: Thin-section computed tomography findings and histopathologic comparisons. J Comput Assist Tomogr. 2004;28:776-781.
- Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastases: Spectrum of radiologic findings. Radiographics. 2001;21:403-417.
- Chalaoui J, Grégoire P, Sylvestre J, et al. Pulmonary hyalinizing granuloma: A cause of pulmonary nodules. Radiology. 1984;152:23-26.
- Srinivas S, Dhelaria R, Pai D, et al. Multiple calcified pulmonary nodules: An unusual presentation of rheumatoid lung. Clin Radiol. 2007;62:274-276.
- Brown K, Mund DF, Aberle DR, et al. Intrathoracic calcifications: radiographic features and differential diagnoses. Radiographics. 1994;14:1247-1261.
- Srivatsa SS, Burger CD, Douglas WW. Upper lobe pulmonary parenchymal calcification in a patient with AIDS and Pneumocystis carinii pneumonia receiving aerosolized pentamidine. Chest. 1992;101:266-267.
- Felson B, Schwarz J, Lukin RR, et al. Idiopathic pulmonary ossification. Radiology. 1984;153:303-310.
- Fried ED, Godwin TA. Extensive diffuse pulmonary ossification. Chest. 1992;102:1614-1615.
- Woolley K, Stark P. Pulmonary parenchymal manifestations of mitral valve disease. Radiographics. 1999;19:965-972.
- Gevenois PA, Abehsera M, Knoop C, et al. Disseminated pulmonary ossification in end-stage pulmonary fibrosis: CT demonstration. AJR Am J Roentgenol. 1994;162:1303-1304.
- Levy J, Wilmott RW. Pulmonary hemosiderosis. Pediatr Pulmonol. 1986;2:384-391.
- Hartman TE, Müller NL, Primack SL, et al. Metastatic pulmonary calcification in patients with hypercalcemia: Findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1994;162:799-802.
- Marchiori E, Franquet T, Gasparetto TD, et al. Consolidation with diffuse or focal high attenuation: Computed tomography findings. J Thorac Imaging. 2008;23:298-304.
- Chong S, Lee KS, Chung MJ, et al. Pneumoconiosis: comparison of imaging and pathologic findings. Radiographics. 2006;26:59-77.
- Graham CM, Stern EJ, Finkbeiner WE, et al. High-resolution CT appearance of diffuse alveolar septal amyloidosis. AJR Am J Roentgenol. 1992;158:265-267.
- Ohdama S, Akagawa S, Matsubara O, et al. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J. 1996;9:1569-1571.
- Ward S, Heyneman LE, Reittner P, et al. Talcosis associated with IV abuse of oral medications: CT findings. AJR Am J Roentgenol. 2000;174:789-793.
- Akira M, Kozuka T, Yamamoto S, et al. Inhalational talc pneumoconiosis: radiographic and CT findings in 14 patients. AJR Am J Roentgenol. 2007;188:326-333.
- Bendeck SE, Leung AN, Berry GJ, et al. Cellulose granulomatosis presenting as centrilobular nodules: CT and histologic findings. AJR Am J Roentgenol. 2001;177:1151-1153.
- Marchiori E, Gonçalves CM, Escuissato DL, et al. Pulmonary alveolar microlithiasis: High-resolution computed tomography findings in 10 patients. J Bras Pneumol. 2007 Oct;33:552-557.
- Gasparetto EL, Tazoniero P, Escuissato DL, et al. Pulmonary alveolar microlithiasis presenting with crazy-paving pattern on high resolution CT. Br J Radiol. 2004;77:974-976.
- Tachibana T, Hagiwara K, Johkoh T. Pulmonary alveolar microlithiasis: Review and management. Curr Opin Pulm Med. 2009;15:486-490.
- Kuhlman JE, Teigen C, Ren H, et al. Amiodarone pulmonary toxicity: CT findings in symptomatic patients. Radiology. 1990;177:121-125.
- Yüksel M, Akgül AG, Evman S, et al. Suture and stapler granulomas: A word of caution. Eur J Cardiothorac Surg. 2007;31:563-565.