May-Thurner Syndrome
Images
Case Summary
An adolescent female presented to the emergency department with a chief complaint of left lower-extremity swelling and pain for three days. A detailed medical history revealed that the patient was taking oral contraceptive pills but was otherwise unremarkable. On physical assessment, the entire left leg was edematous, warm, and painful on palpation. The patient was referred to interventional radiology for thrombolysis and possible stent placement.
Imaging Findings
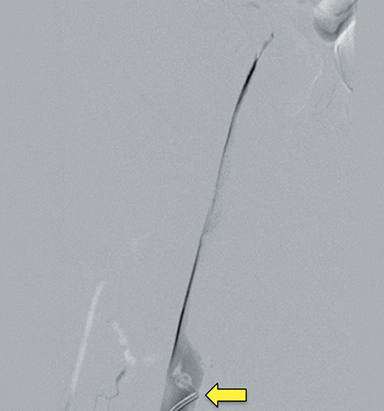
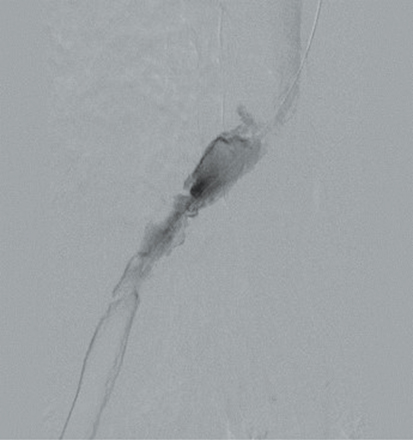
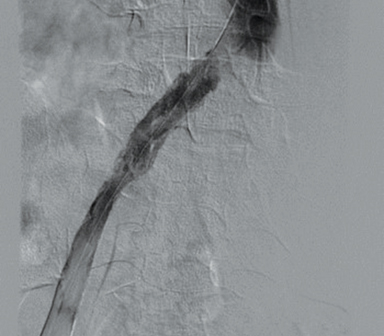
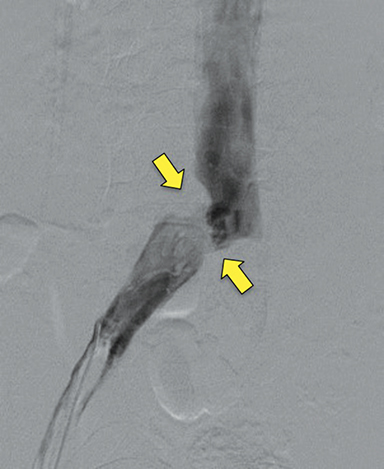
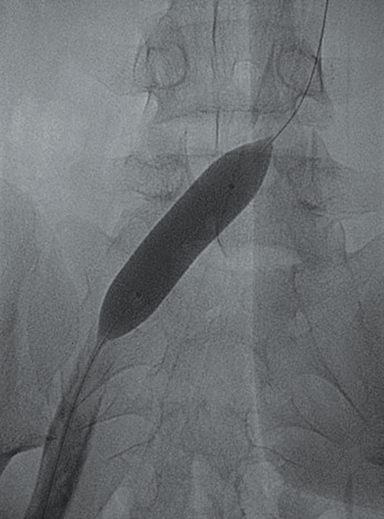
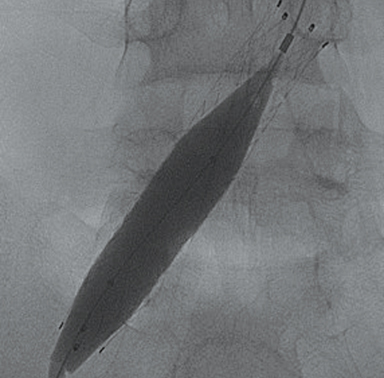
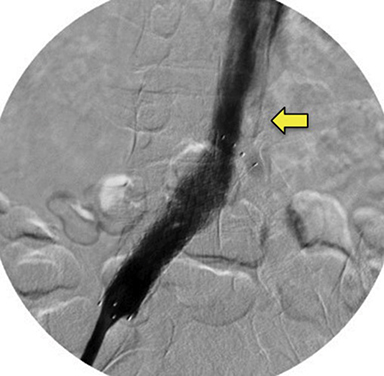
Doppler ultrasound of the leg (not shown) demonstrated occlusive thrombus within the left external and common iliac veins (Figure 1). Catheter venography was performed via the left popliteal vein with the patient in the prone position. The catheter tip, positioned in the left external iliac vein, demonstrated a near-completely occlusive thrombus extending from the left external iliac vein to the left common iliac vein with minimal central venous drainage into the IVC (Figure 2). Catheter-directed chemical thrombolysis with tissue plasminogen activator (tPA) was then performed to reduce or eliminate the clot burden and re-establish venous blood flow. A pulse spray catheter was inserted and positioned within the clot for tPA infusion. Repeat venography 24 hours after initiation of tPA infusion demonstrated minimal residual thrombus and a persistent narrowing of the left common iliac vein immediately proximal to its drainage into the IVC, confirming diagnosis of May-Thurner syndrome. A percutaneous transluminal angioplasty (PTA) was then performed (Figure 3) with a 14 mm by 4 mm balloon centered along the site of narrowing and inflated to 8 ATM. After PTA there was persistent stenosis. Therefore, a 14 mm x 40 mm wall stent was placed across the iliocaval stenosis. Lastly, a post-stent placement venogram was performed, demonstrating non-obstructed antegrade blood flow into the IVC.
Diagnosis
May-Thurner syndrome
Discussion
May-Thurner syndrome (MTS), first described by May and Thurner in 1957, is a rare pathological condition in which the left common iliac vein is compressed by the overriding right iliac artery. MTS is commonly associated with left lower-extremity deep venous thrombosis (DVT). In fact, MTS is thought to represent 2-5% of all lower extremity DVTs.1 Other hypercoagulable states such as immobilization, trauma, and obesity, in addition to genetic factors such as Factor V Leiden mutations, also present risk factors for the development of MTS and concomitant left lower-extremity DVT. MTS is seen most commonly in pregnancy and the postpartum period, typically presenting in the second to fourth decades of life. The condition is also seen in men; however, it is three times more common in women, owing to marked increase in hypercoagulability in the pregnancy/postpartum periods. In pediatric populations, MTS has been associated with inherited thrombophilia and maturation of the coagulation system. In a report describing three pediatric adolescent cases of MTS,2 each patient had additional acquired risk factors for thrombosis. One patient was obese and had a diagnosis of nephrotic syndrome, another patient was also obese and had antiphospholipid antibody syndrome, and the third patient had type III Protein S deficiency. Additionally, all three patients were taking oral contraceptive pills, as in our patient’s case. A separate report by Eng, et al, describes a case of a kidney causing deep venous thrombosis via direct compression of the iliac veins in a pediatric patient with no hereditary risk factors for thrombophilia.3
A similar pathology of venous obstruction resulting from overlying anatomical structures is seen in nutcracker syndrome (NCS), or left renal vein entrapment syndrome. This condition describes a compression of the left renal vein (LRV). The most common type of NCS is anterior NCS or a compression of the LRV between the aorta and superior mesenteric artery (SMA). Compression of the LRV obstructs the flow of blood and causes a subsequent distention of the distal portion of the vein, leading to elevated pressure in the LRV. This increase in pressure leads to congestion of the left kidney and the development of collateral veins. In posterior NCS, the retroaortic or circumaortic renal vein is compressed between the aorta and a vertebral body. Children presenting with NCS are largely asymptomatic, but flank/abdominal pain and varicocele may be present; urinalysis findings of proteinuria and hematuria are also common. Treatment often includes renal vein stenting and revascularization, although in many patients regular follow-up is sufficient.
The pathophysiology of MTS results from continuous mechanical injury to the endothelial cells of the left common iliac vein as a result of the pulsations from the right iliac artery.1 The constant stimulation causes the collagen within the endothelium of the left common iliac vein to become rough with spurs, leading to accumulation of elastin and resulting in hyperplasia of the blood vessel with stenosis.1 This intimal hyperplasia in the setting of Virchow Triad (hypercoagulability, stasis of blood flow, and endothelial injury) creates a “perfect storm” for the development of a DVT (Figure 2). This risk becomes even more of an issue during pregnancy, when the overall risk of venous thromboembolism (VTE) increases four- to fivefold compared to the non-pregnant state.4 The risk of DVT is 20 to 80 times higher during the postpartum period than during pregnancy, 5 and the risk further multiplies to 100 times higher in the first week postpartum.6 During pregnancy, especially in the third trimester, compression of the left iliac vein by the enlarged uterus presents yet another risk factor for MTS development.1
Clinically, symptoms of MTS are consistent with those of venous insufficiency and DVT; they include swelling with or without pain of the left lower extremity, enlarged veins, feelings of increased warmth in the leg, and erythema/other discoloration of the skin. Ultrasound is typically the first-line diagnostic imaging modality, given its accessibility and noninvasive nature. However, the gold standard for confirmatory diagnosis remains contrast venogram, which is able to demonstrate narrowing of iliac vein at the level of the pelvic inlet.7 With advancements in CT scanning techniques and quality of imaging, however, the non-invasive CT venogram also offers diagnostic ability comparable to the catheter venogram. A 2015 study by Kuo, et al, suggests that both CT venogram and digital subtraction venogram provide the essential information required for evaluation and diagnosis of MTS.7
Treatment of MTS depends on whether a DVT is present. In the absence of DVT in patients with no or mild symptoms, conservative treatment with compression stockings and possibly anticoagulation therapy is recommended.8 For advanced non-thrombotic MTS with signs and/or symptoms of chronic venous insufficiency (skin discoloration, limb swelling, and pain) treatment with angioplasty and stenting of the affected segment is recommended to reduce the severity of venous stenosis.9 In cases exhibiting thrombotic venous occlusion, patients are anticoagulated and either catheter-directed thrombolysis or pharmacomechanical thrombolysis is recommended. If underlying intrinsic venous stenosis is present, angioplasty and stenting are also indicated.10 Mechanical thrombolysis with suction thrombectomy is recommended for patients with contraindications to lytic therapy.10 Post-thrombotic syndrome is a complication associated with venous thrombotic occlusion and MTS. In general, post-thrombotic syndrome refers to chronic venous insufficiency resulting from reflux due to valvular incompetence combined with venous hypertension.11 Successful treatment of MTS results in post-thrombotic syndrome rates of less than 10 percent. Without treatment, post-thrombotic syndrome rates are as high as 80 to 90 percent.12
Conclusions
May-Thurner syndrome, though rare, should be considered in the differential diagnosis, especially in pregnant/postpartum women, in the setting of suspected lower extremity DVT. Its diagnosis and treatment reduce complications such as post-thrombotic syndrome, pulmonary embolism, and death. Modern minimally invasive treatments such as catheter thrombolysis and thrombectomy are safe options that demonstrate high rates of success.
References
- Inam HA, Shaikh FA, Siddiqui N. May-Thurner syndrome: A cause of acute left iliac vein obstruction in early postpartum period: a case report. J Pakistan Med Assoc. 2019;69(7). https://jpma.org.pk/article-details/9246?article_id=9246. Accessed February 1, 2020.
- Raffini L, Raybagkar D, Cahill AM, Kaye R, Blumenstein M, Manno C. May–Thurner syndrome (iliac vein compression) and thrombosis in adolescents. Pediatric Blood & Cancer. 2005;47(6):834-838. doi:10.1002/pbc.20728.
- Eng JM, Walor DM, Michaels LA, Weiss AR. An unusual presentation of May–Thurner Syndrome in a pediatric patient with a pelvic kidney. J Ped Urol. 2013;9(1). doi:10.1016/j.jpurol.2012.08.014.
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJM. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haem. 2008;6(4):632-637. doi:10.1111/j.1538-7836.2008.02921.x.
- Duran C, Rohatgi S, Wake N, Rybicki FJ, Steigner M. May-Thurner syndrome: A case report. Eurasian J Med. 2011;43(2):129-131. doi:10.5152/eajm.2011.29.
- James AH. Pregnancy-associated thrombosis. Hematology. 2009;2009(1):277-285. doi:10.1182/asheducation-2009.1.277.
- Kuo Y-S, Chen C-J, Chen J-J, et al. May-Thurner syndrome: Correlation between digital subtraction and computed tomography venography. J Formosan Med Assoc. 2015;114(4):363-368. doi:10.1016/j.jfma.2012.12.004.
- Hager ES, Yuo T, Tahara R, et al. Outcomes of endovascular intervention for May-Thurner syndrome. J Vasc Surg: Venous Lymph Dis. 2013;1(3):270-275. doi:10.1016/j.jvsv.2012.11.002.
- Igari K, Kudo T, Toyofuku T, Jibiki M, Inoue Y. Surgical thrombectomy and simultaneous stenting for deep venous thrombosis caused by iliac vein compression syndrome (May-Thurner Syndrome). Ann Thoracic Cardiovasc Surg. 2014;20(6):995-1000. doi:10.5761/atcs.oa.13-00213.
- Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192(6):782-788. doi:10.1016/j.amjsurg.2006.08.045.
- Wang W, Sun R, Chen Y, Liu C. Meta-analysis and systematic review of percutaneous mechanical thrombectomy for lower extremity deep vein thrombosis. J Vasc Surg: Venous Lymph Dis. 2018;6(6):788-800. doi:10.1016/j.jvsv.2018.08.002.
- Bergan JJ, Schmid-Schönbein GW, Smith PDC, Nicolaides AN, Boisseau MR, Eklof B. Chronic Venous Disease. NEJM. 2006;355(5):488-498. doi:10.1056/nejmra055289.
References
Citation
AN D, RB T, AJ T, Schaefer, CM, DJ A. May-Thurner Syndrome. Appl Radiol. 2021;(4):56A-56D.
July 15, 2021