Metastatic adenocarcinoma involving atypical meningioma
Images

CASE SUMMARY
A 66-year-old female with a history of stage IV lung adenocarcinoma presented to the emergency department with aphasia. The patient noted early morning language deficits with maintained comprehension. One week prior to this, a similar episode occurred that lasted 20 minutes. The patient was alert and oriented with expressive aphasia. Facial symmetry, sensation, and strength were intact. An EEG was performed and was negative for seizures with mild nonspecific diffuse encephalopathy noted. A head CT was performed, which revealed a previously seen left middle cranial fossa meningioma and associated edema. The patient was managed with anti-seizure medications and steroids, and was discharged the next day. Plans were made to follow up with neurosurgery for surgical resection five days later.
IMAGING FINDINGS
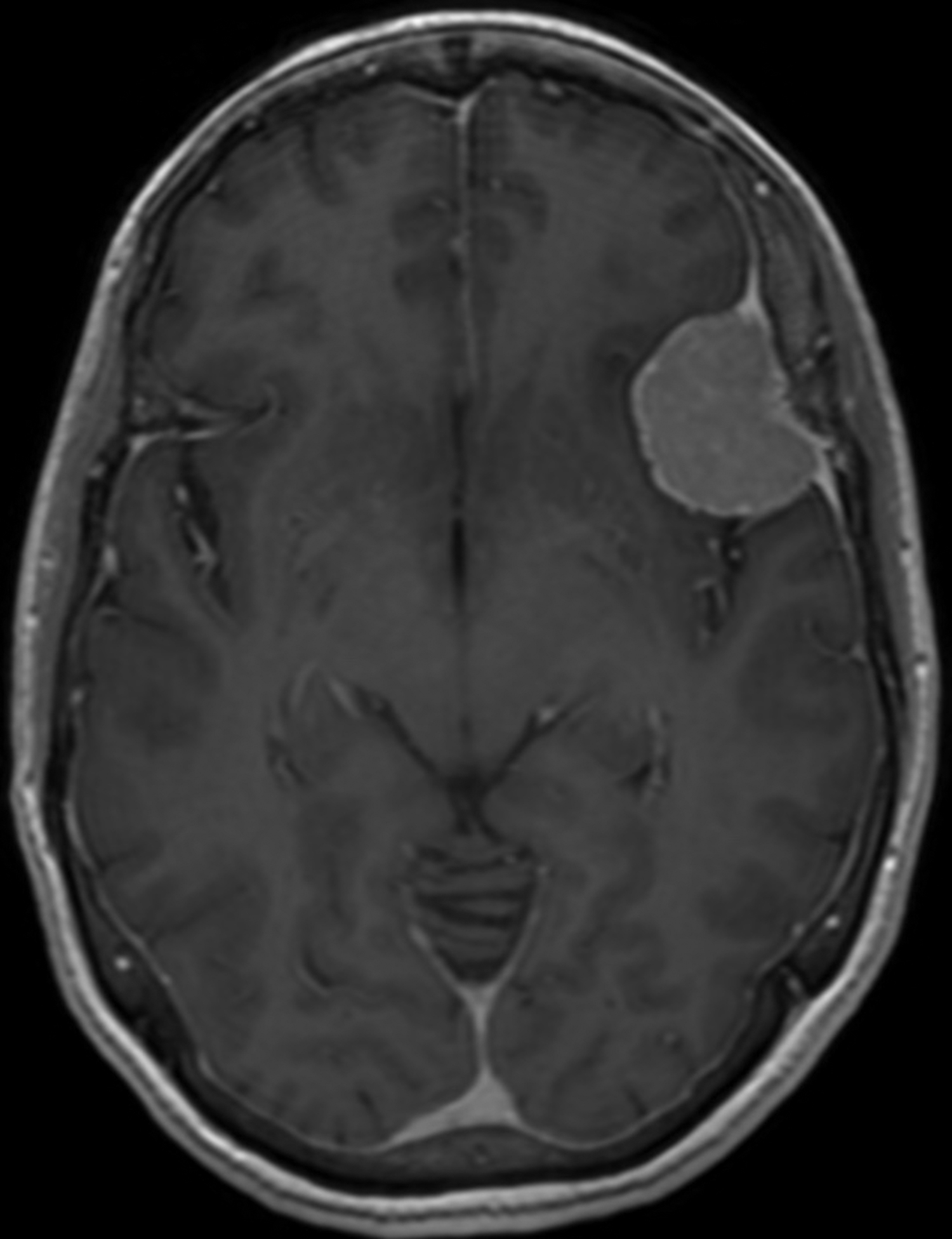
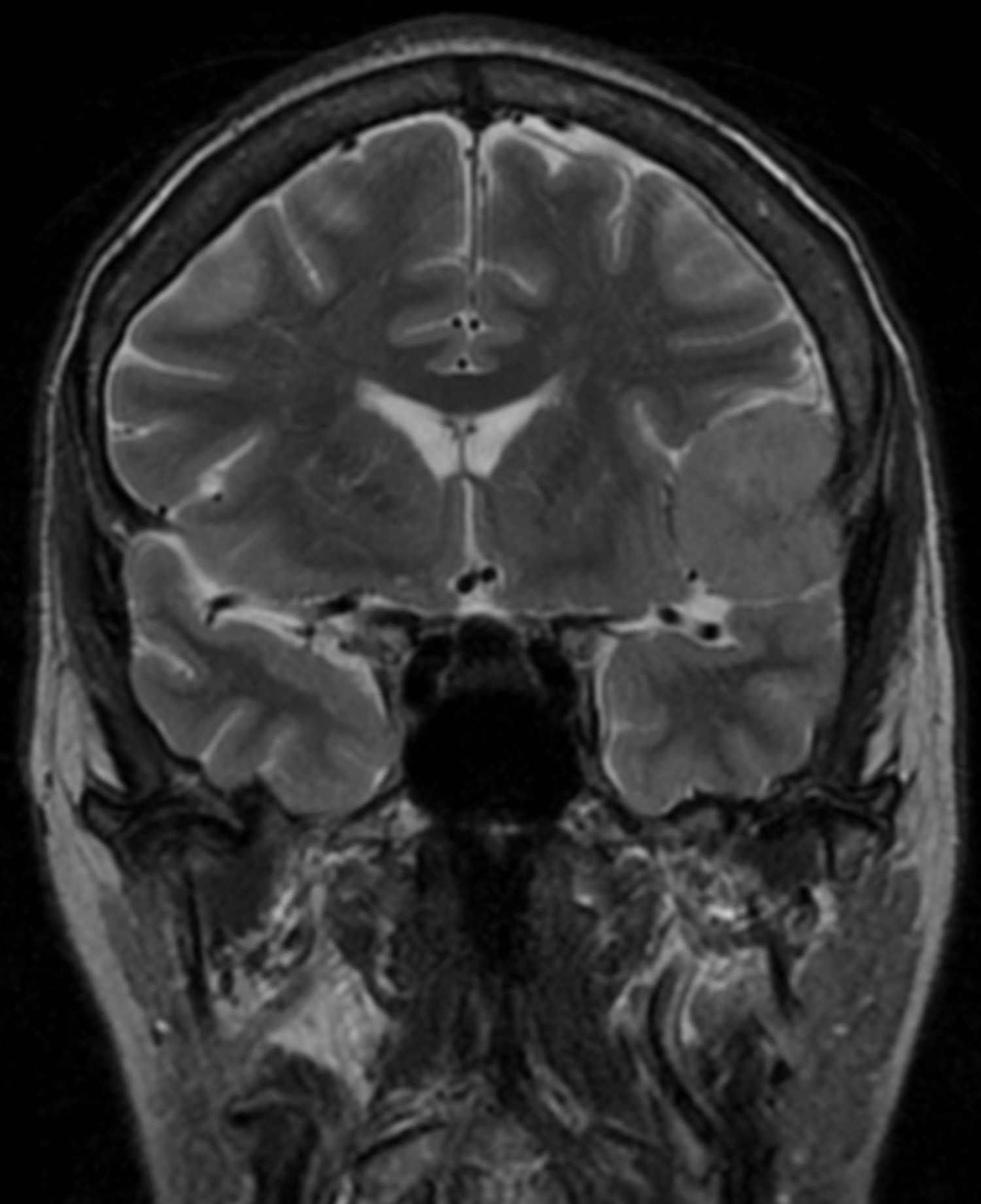
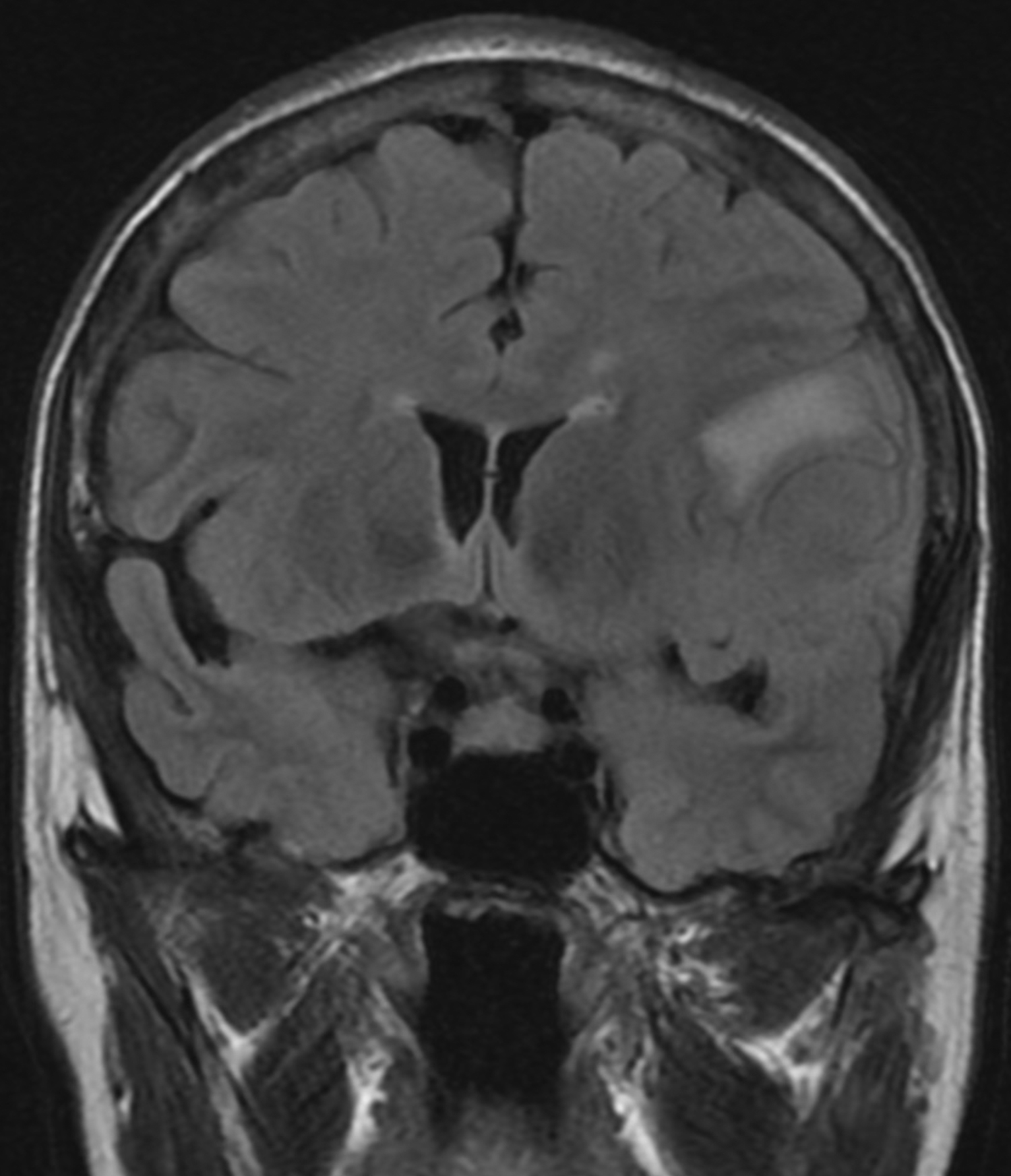
A head MRI performed two weeks prior to the presentation (for lung cancer staging) showed a left middle cranial fossa, 3.9 × 3.7 × 2.9 cm contrast enhancing, extra-axial, and dural based mass, with possible mineralization centrally within the mass (Figure 1C). Two years prior, this mass measured 3.0 × 2.9 × 2.5 cm on MRI (Figure 1A and B). There continued to be mild mass effect on the adjacent brain parenchyma and frontal horn of the left lateral ventricle, and there were new zones of mild, vasogenic edema seen adjacent to the mass (Figure 1D).
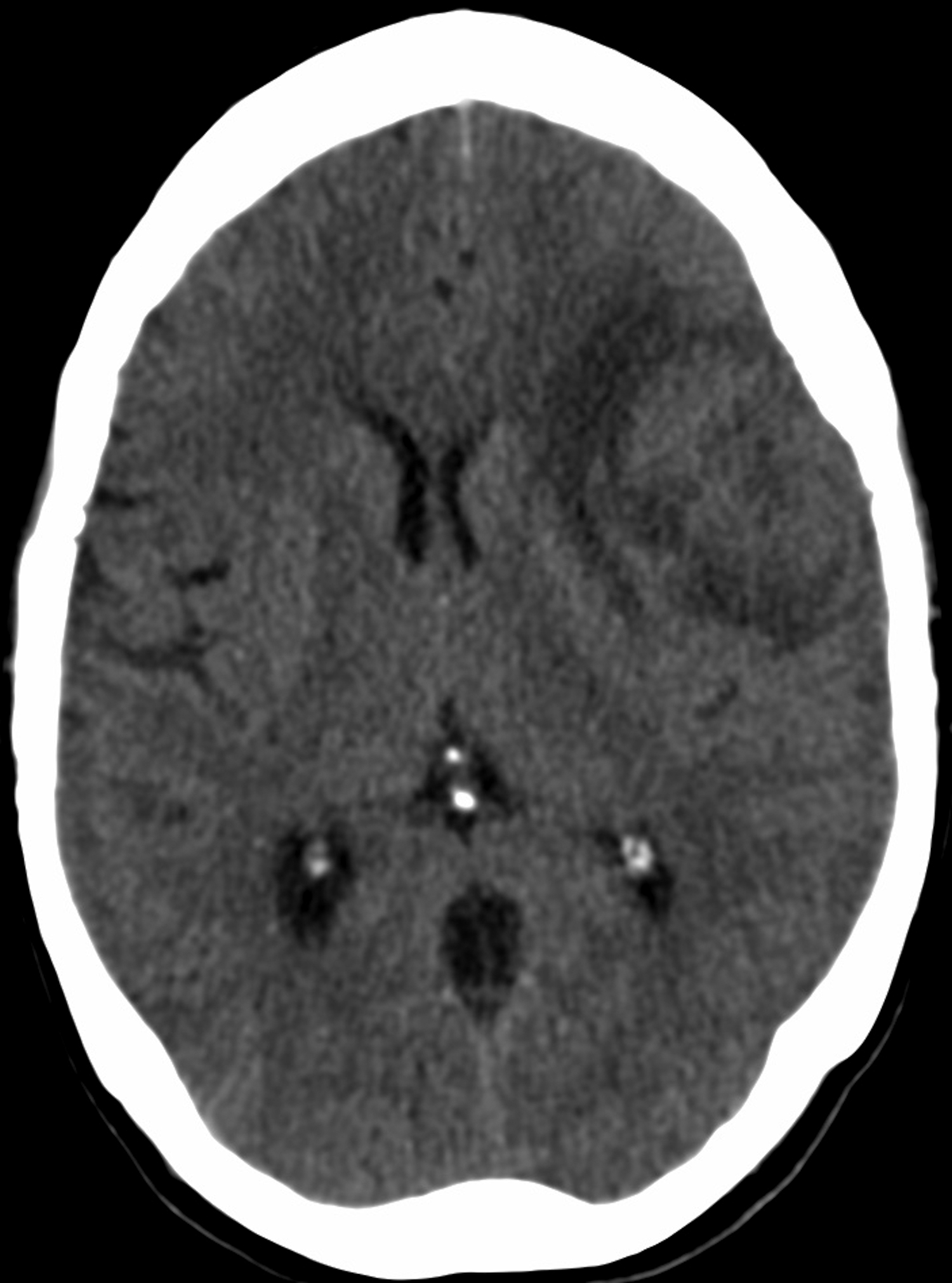
CT of the head without contrast performed during the admission similarly showed the left middle cranial fossa lesion with associated edema, a significant size increase, increased mass effect, midline shift of 3 mm, and presumed necrosis (Figure 1E).
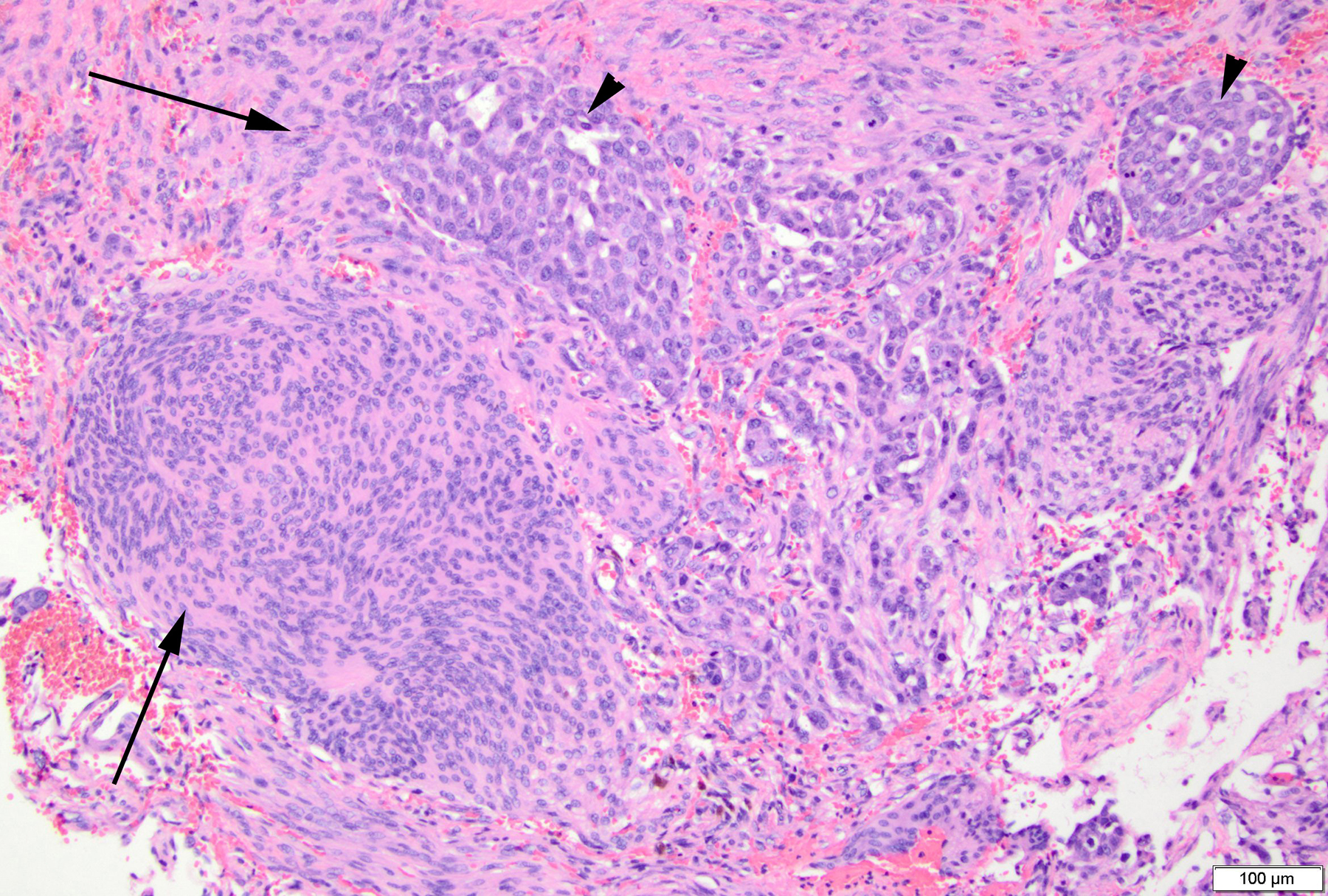
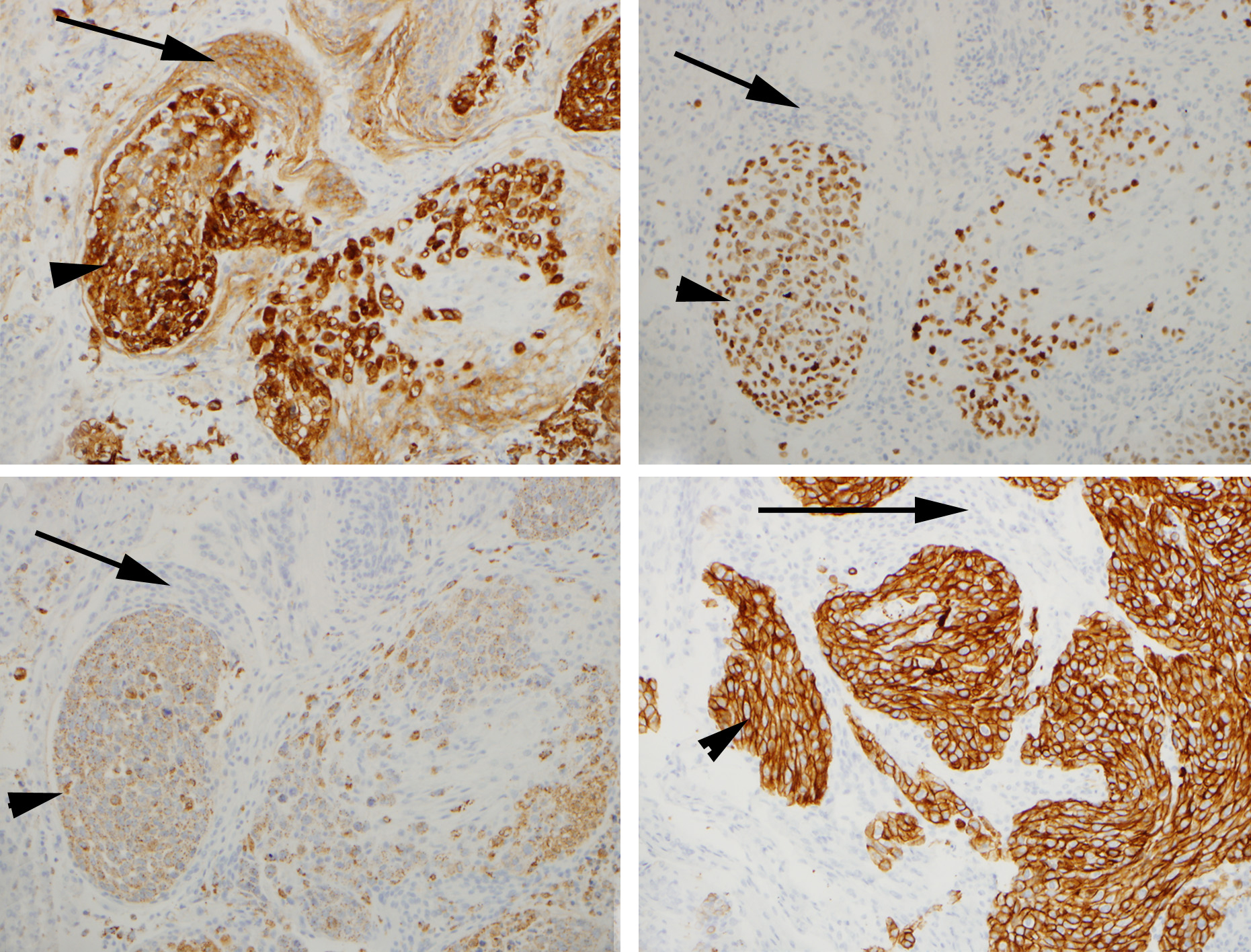
Histologically, the dural-based mass was a meningioma with a syncytial meningothelial pattern. However, atypical features were present and included necrosis, loss of lobular pattern, small cell change, and hypercellularity. Additionally, this was involved by a metastatic adenocarcinoma with gland formation, mucin, high mitotic activity, and necrosis (Figure 2). Immunohistochemical (IHC) stains were performed and EMA was positive in the meningioma as well as the adenocarcinoma, while pulmonary origin of the adenocarcinoma was confirmed with additional stains for TTF1, Napsin A, and CK7 (Figure 3). Radiologic differential diagnostic considerations include meningioma, a higher-grade meningioma, metastasis, or a tumor-to-tumor metastasis.
DIAGNOSIS
Metastatic adenocarcinoma of pulmonary origin involving an atypical meningioma, World Health Organization (WHO) grade II
DISCUSSION
Meningiomas consist of neoplastic meningothelial cells (arachnoidal) and represent approximately 36 percent of all intracranial neoplasms that usually occur in the sixth-seventh decade.1 Most commonly these occur intracranially including at the cerebral convexities, sphenoid ridges, and petrous ridges.1 There is a gender predilection for females; however, in grade II and grade III lesions, males are more commonly affected.1 Presentation is nonspecific and may include seizures and headache.1
Meningiomas are divided into three groups per WHO criteria based on morphologic features, including grade I, II, and III. Grade I meningiomas lack any higher grade histological features and have many differing histologic patterns including meningothelial (classic pattern), fibrous, transitional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, and metaplastic.1 Grade II meningiomas, deemed atypical meningiomas include those with a clear cell or chordoid histologic pattern as well as any lesion that displays brain invasion or ≥ 4 mitoses per high power field (hpf).1,2 Lastly, evidence of ≥ 3 of the following five parameters including macronucleoli, sheeting (loss of lobular architecture), necrosis, small cell change, and hypercellularity are also diagnostic of an atypical meningioma.1,2 Grade III meningiomas, deemed anaplastic meningiomas include those with a papillary or rhabdoid histologic pattern as well as any lesion that displays > 20 mitoses/hpf or that shows evidence of carcinomatous/sarcomatous elements.1
Tumor to tumor metastases within meningiomas are a rare occurrence with just about 100 cases reported in the literature.3 The most common meningioma type that a lesion is metastatic to includes the meningothelial subtype while the most common metastatic lesions to a meningioma include breast followed by lung.3,4 Our case demonstrates the first atypical meningioma reported with a metastasis to our knowledge.3-5 Histologic features meeting a true “tumor to tumor metastasis” versus a collision tumor as previously described were also met in this case.6,7 Postulations as to why meningiomas are the most frequent intracranial neoplasm harboring metastases include slow indolent growth, hypervascularity, high collagen and lipid content, and a general noncompetitive environment.3-5 Presentation of a primary neoplasm metastasizing to a meningioma has also been reported.4,8
CT and MRI imaging are unable to definitively diagnose a metastasis within a meningioma.3 However, atypical MRI findings, such as the peritumoral edema seen in our case, may suggest the possibility of a metastasis within a meningioma.3,4 Peritumoral brain edema associated with a meningioma may also suggest a higher-grade lesion or differing variant.9 Perfusion MR and MR spectroscopy have been suggested to be useful in distinguishing higher grades or differing variants of meningiomas.3
CONCLUSION
Metastases to meningiomas are relatively rare. Atypical image findings on MRI may include increased edema, which should raise suspicion of another process. Even without any clinical history of a primary neoplasm this process should be considered and evaluated by pathology.
REFERENCES
- Louis D, Ohgaki H, Wiestler O, et al., (eds). World Health Organization Classification of Tumours of the Central Nervous System. Lyon: IARC Press, 2016.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta neuropathologica. 2016;131:803-820.
- Moody P, Murtagh K, Piduru S, et al. Tumor-to-tumor metastasis: pathology and neuroimaging considerations. Int J Clin Exp Pathol. 2012 Jan 1;5(4):367-373.
- Bhargava P, McGrail KM, Manz HJ, Baidas S. Lung carcinoma presenting as metastasis to intracranial meningioma: case report and review of the literature. American journal of clinical oncology. 1999 Apr 1;22(2):199-202.
- Takei H, Powell SZ. Tumor-to-tumor metastasis to the central nervous system. Neuropathology. 2009 Jun 1;29(3):303-308.
- Campbell LV Jr, Gilbert E, Chamberlain CR Jr, Watne AL. Metastases of cancer to cancer. Cancer 1968; 22: 635-643.
- Pamphlett R. Carcinoma metastasis to meningioma. J Neurol Neurosurg Psychiatry 1984; 47: 561-563.
- Caroli E, Salvati M, Giangaspero F et al. Intrameningioma metastasis as first clinical manifestation of occult primary breast carcinoma. Neurosurg Rev 2006;29: 49-54.
- Osawa T, Tosaka M, Nagaishi M, Yoshimoto Y. Factors affecting peritumoral brain edema in meningioma: special histological subtypes with prominently extensive edema. J Neurooncol. 2013 Jan 1;111(1):49-57.
Citation
D A, P R, G Y. Metastatic adenocarcinoma involving atypical meningioma. Appl Radiol. 2018;(3):27-28.
February 9, 2018