MRI in pregnancy: Gastrointestinal and genitourinary pathology
Images
The presentation of abdominal pain in pregnancy is a diagnostic challenge for clinicians. Often, intra-abdominal pathology in pregnancy can be masked by maternal physiologic and anatomic changes including leukocytosis, displacement of abdominal organs by a gravid uterus, nausea and vomiting, and a difficult physical exam.1,2 When true pathology does exist, a delayed diagnosis can lead to unfavorable outcomes for both mother and fetus. In the setting of a confusing clinical picture, imaging is a crucial tool for the evaluation of pregnant patients with abdominal pain.
Magnetic resonance imaging (MRI) is beneficial in pregnancy as it allows for excellent soft tissue contrast resolution and for the evaluation of multiple organ systems without exposure to ionizing radiation.3 In this review, we will discuss the safety of MRI in pregnancy and our institution’s technique for imaging pregnant patients with abdominal pain by MRI. We will focus on the MRI appearance of common abdominal pathologies seen in pregnancy with attention to the gastrointestinal and genitourinary systems.
MRI technique
The American College of Radiology (ACR) approves the use of MRI in pregnancy. Although MRI is performed without ionization radiation, radiofrequency pulses utilized in MRI deposit energy in patients in the form of heat. The amount of energy deposited is referred to as the specific absorption rate (SAR) and is monitored on all modern MRI systems. The predicted fetal temperature rise caused by this energy deposition, to this point, has never been demonstrated to cause fetal teratogenicity in any trimester of pregnancy.4 MRI in pregnancy is, therefore, felt to be safe in all trimesters. As with any intervention in pregnancy, however, the ACR stresses that a risk-benefit analysis be performed, and in our institution, informed consent is always obtained in pregnant patients prior to undergoing MRI. If an MRI is deemed non-urgent, the study should be delayed until the patient is no longer pregnant.4
The ACR recommends against the routine use of MRI contrast agents in pregnant patients. Studies have demonstrated that at least some of the gadolinium chelate traverses the placenta and may accumulate in the amniotic cavity, with contrast cycling through the fetal gastrointestinal and genitourinary tracts for an indefinite period of time.5 Although the risk of using gadolinium in pregnancy remains unknown, there is a potential for chelate dissociation and the formation of toxic gadolinium ions.4,5,6 The decision to use contrast should therefore be made after a risk-benefit analysis and on a case-to-case basis.
At our institution, pregnant patients are imaged supine on a 1.5 T magnet with a phased-array body coil. No intravenous or oral contrast agent is administered. Image coverage extends from the superior aspect of the gallbladder through the pelvis. Key components of our protocol are multiplanar, T2-weighted steady-state fast spin echo (SSFSE) sequences, which are useful for localization of anatomic structures and for highlighting edema and fluid, particularly when fat-suppression technique is applied. Axial T1-weighted in-phase and opposed-phase gradient echo imaging is performed for soft tissue characterization, including identifying the presence of bulk or microscopic fat, hemorrhage or protein, and susceptibility artifact, which can aid in the identification of a normal air-containing appendix. Diffusion-weighted imaging can highlight areas of inflammation, infection, or neoplasm, and provide value, especially in the setting of a noncontrast study. Finally, 2D time-of-flight imaging is utilized to help differentiate a normal appendix from other tubular mimickers such as pelvic varices.
Gastrointestional pathologies
While the most common cause of acute abdominal pain in pregnancy is appendicitis, other frequently encountered gastrointestinal causes include bowel obstruction and inflammatory bowel conditions.7
Appendicitis
Appendicitis is the most common non-obstetric cause for emergency surgery in pregnancy.8 Acute appendicitis in pregnancy, particularly perforated appendicitis, has been linked to premature labor and maternal death, making early diagnosis essential.9 Studies have demonstrated that MRI can reliably diagnose acute appendicitis during pregnancy with sensitivity and specificity of 100% and 94%, respectively.3,9
Localization of the appendix can be challenging in pregnant patients due to changes in abdominal organ position caused by the gravid uterus. One means to localize the appendix is a cecal tilt angle of at least 90° or greater on sagittal T2-weighted imaging, which predicts localization of the appendix to the right upper quadrant regardless of gestational age.10 MR imaging features of a normal appendix include a diameter of less than 6 mm, a collapsed or partially air-filled lumen, and lack of periappendiceal stranding. T1-weighted in-phase and opposed-phase gradient echo imaging is especially useful for identifying luminal gas in the appendix, seen as susceptibility artifact on the longer echo time in-phase sequence compared to the opposed-phase sequence. When gas is seen throughout the appendiceal lumen, the diagnosis of appendicitis is essentially excluded, regardless of appendix diameter.
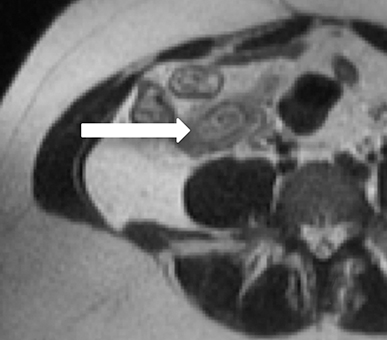
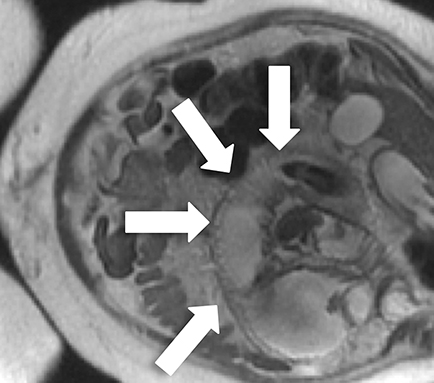
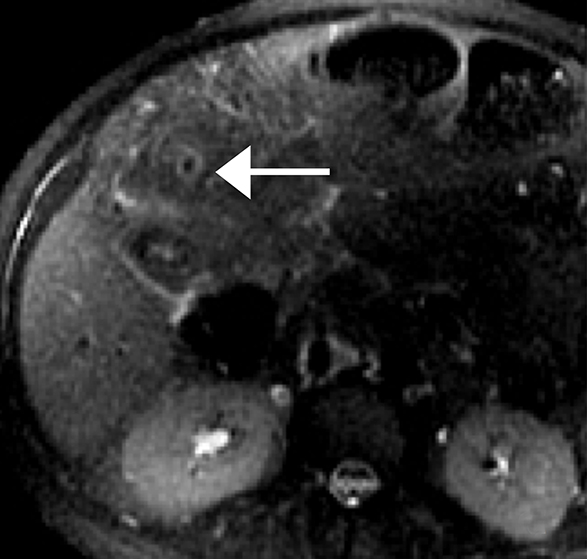
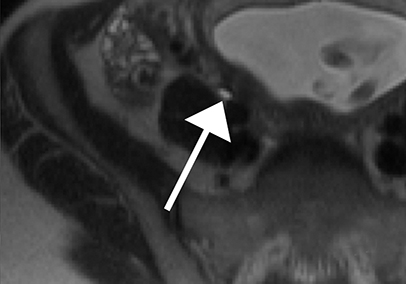
Features of acute appendicitis on MRI include a dilated appendix greater than 7 mm in diameter, hyperintense intraluminal fluid on T2-weighted imaging, and a thickened, edematous wall appearing brighter on T2-weighted images compared to bowel wall elsewhere. Restricted diffusion can also be seen in acute appendicitis, and when apparent, increases the sensitivity for accurate diagnosis.11 Secondary signs of appendicitis include periappendiceal inflammation appearing as hyperintense signal on T2-weighted images made particularly apparent with the use of fat-suppression techniques (Figure 1). Appendicoliths, when present, may appear as focal hypointense structures on T1- and T2-weighted imaging and can demonstrate susceptibility artifact.
Bowel obstruction
Bowel obstruction in pregnancy has an incidence of approximately 1 in 1,500 to 1 in 66,000.12 The frequency of mechanical obstruction increases with older gestational age.13 Common symptoms of pregnancy including nausea, vomiting, and constipation mimic those symptoms classic for bowel obstruction, making diagnosis a challenge.
Causes of obstruction are similar to those seen in non-pregnant patients, with adhesions being most common, accounting for 58% of obstructions in pregnant patients.14 Volvulus is the second most common cause of obstruction in pregnancy occurring in approximately 25% versus only 3-5% of cases in non-pregnant patients.15, 16 A unique cause of bowel obstruction in pregnancy results from bowel compression by a gravid uterus.17
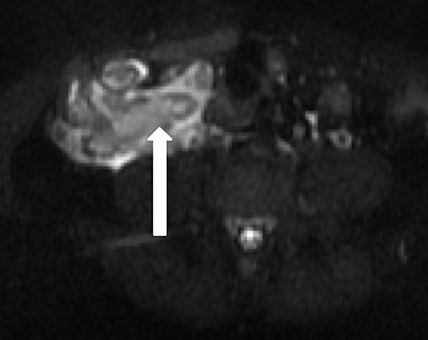
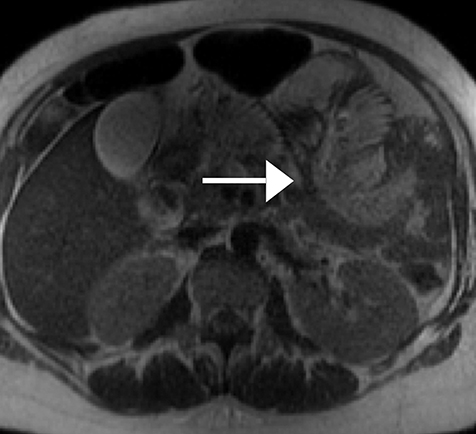
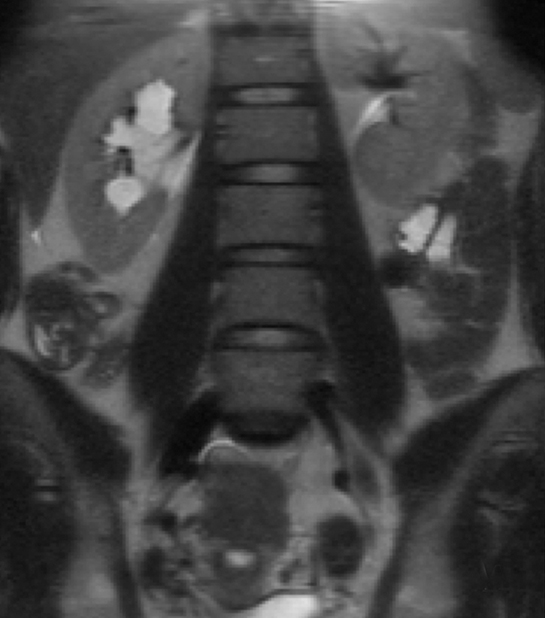
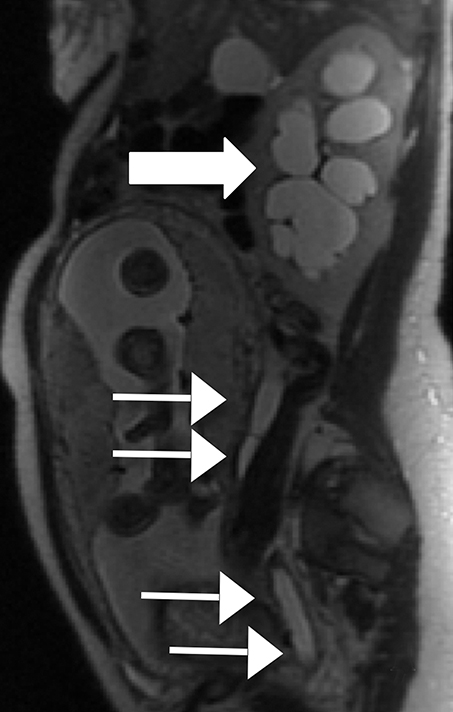
MRI is excellent for the evaluation of bowel obstruction and can be performed without oral or IV contrast. Although motion caused by amniotic fluid, maternal breathing, and peristalsis may limit the evaluation of maternal bowel on MRI, utilization of fast acquisition imaging techniques helps compensate for this limitation. Multiplanar T2-weighted SSFSE imaging has demonstrated particular utility in evaluating the severity of obstruction and identifying the transition point. Obstruction on MRI appears as dilated loops of bowel, often fluid-filled, leading to a transition point, with decompressed downstream bowel loops. Secondary signs, which can help determine the severity of obstruction, such as bowel wall edema, mesenteric edema, and ascites are also well depicted by MRI (Figure 2).
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a common cause of abdominal pain in pregnancy both in women who already carry the diagnosis and those who do not, as both the age of presentation for Crohn’s disease and ulcerative colitis (UC) overlaps with reproduction. IBD often mimics other surgical conditions in pregnancy. For example, Crohn’s disease frequently affects the terminal ileum, making it a common mimicker of acute appendicitis in pregnancy.1
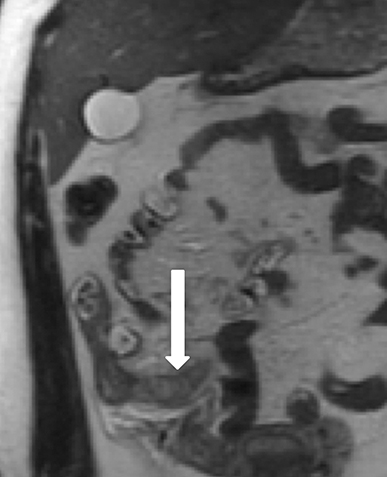
On MRI active Crohn’s disease appears as segments of circumferentially thickened, edematous bowel often with mural stratification on T2-weighted images, frequently with skip lesions and involvement of the terminal ileum (Figure 3). Luminal narrowing is seen both in the acute phase of inflammation, and as a sequela of fibrosis in chronic disease. In addition to stricturing, bowel in chronic disease often demonstrates fatty mural infiltration, well differentiated from wall edema by T2-weighted fat-suppression sequences.
Extraluminal complications of Crohn’s disease such as sinus and/or fistulous tracts, phlegmon, and abscesses are also well evaluated by MRI. The former appear as fluid-filled linear areas of signal abnormality on T2-weighted sequences arising from bowel loops, often with a stellate configuration and associated bowel tethering.18 Phlegmon appears as a mass-like area of hyperintensity on T2-weighted imaging within the mesenteric fat, while abscesses are walled-off extraluminal fluid collections, sometimes containing susceptibility due to the presence of gas.
In contrast to Crohn’s disease, inflammation in UC involves the rectum and spreads proximally in a contiguous manner. Even in the most severe cases, wall thickening in UC is less dramatic then in Crohn’s disease, as inflammation is not transmural and spares the serosal surface. As in acute Crohn’s disease, secondary signs of active inflammation such as free fluid and edema in the surrounding soft tissues are well depicted by MRI. Chronic changes of UC include a featureless, ahaustral colon with fibrofatty proliferation of surrounding soft tissues.
Genitourinary pathologies
Various genitourinary pathologies can manifest as abdominal pain in pregnancy, including obstructing calculi, ovarian torsion, and degenerating fibroids.
Obstructive hydronephrosis
The most frequent cause of obstructive hydronephrosis in pregnancy is urinary tract calculi. Acute urolithiasis is the most common cause of nonobstetric hospital admission during pregnancy.19, 20 Potential complications of urolithiasis include infection and premature labor, highlighting the importance of differentiating between physiologic and obstructive hydronephrosis.
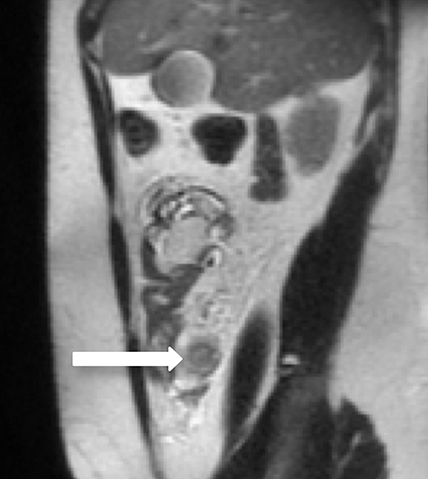
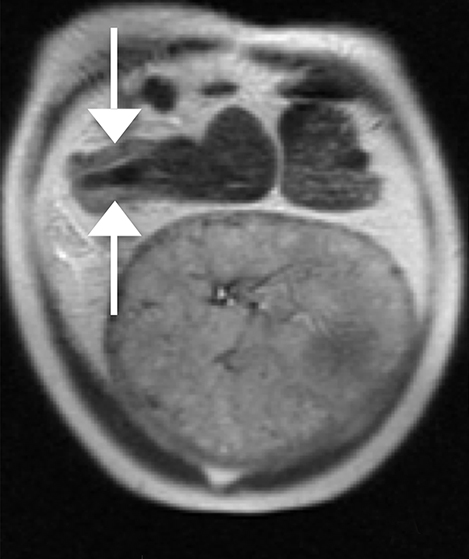
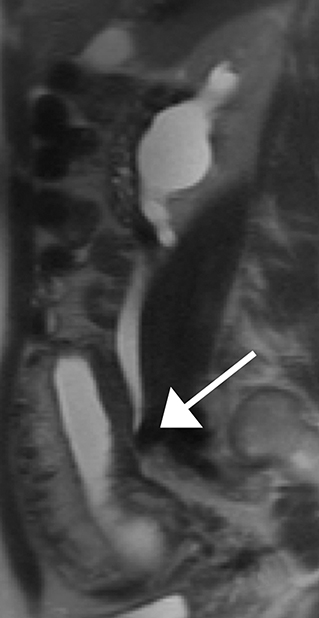
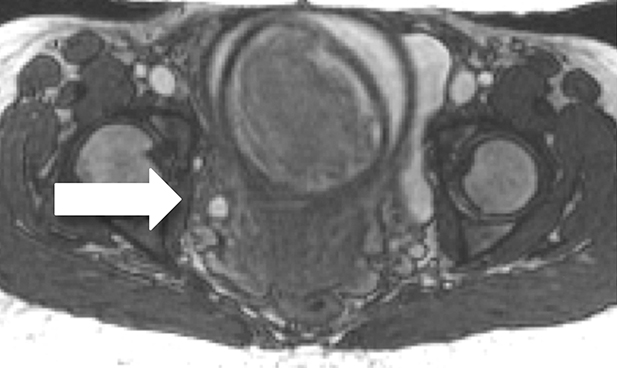
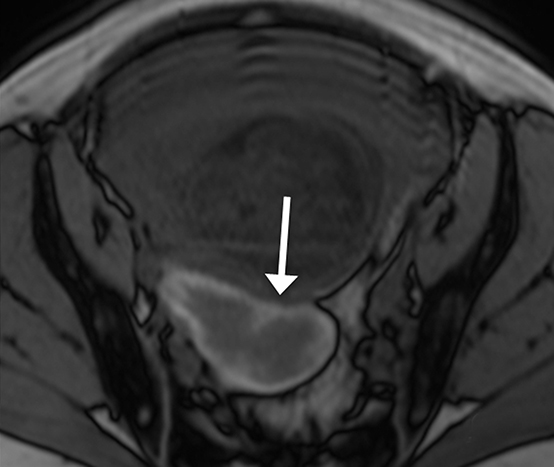
Physiologic hydronephrosis occurs in 90% of pregnant patients and is most often asymptomatic, but has been a reported cause of abdominal pain.21 Physiologic hydronephrosis is caused by a combination of extrinsic compression of the ureter and hormonal-induced ureteral relaxation, occurring most frequently on the right (Figure 4).22 Physiologic hydronephrosis manifests as gradual tapering of the mid/distal ureter due to compression between a gravid uterus and the iliopsoas muscle.23 In physiologic hydronephrosis, no filling defect is seen, the ureter is not dilated distal to the sacral promontory and only rarely is there associated renal enlargement or perinephric fluid.
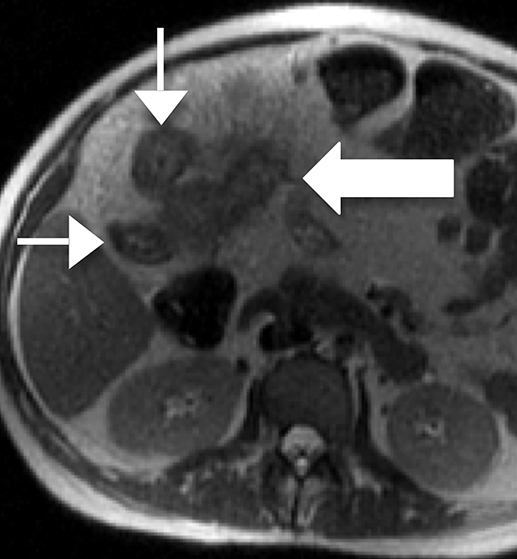
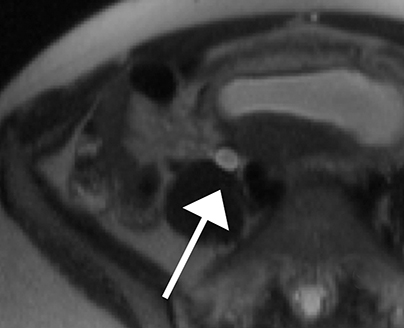
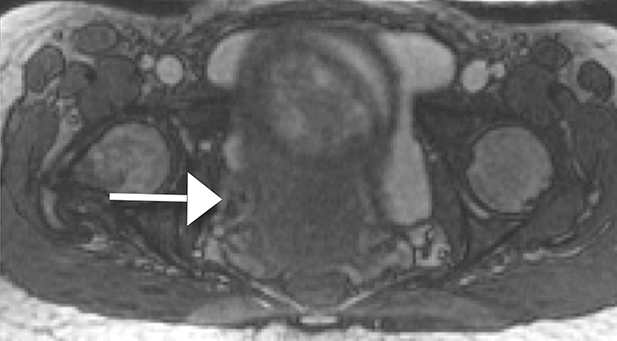
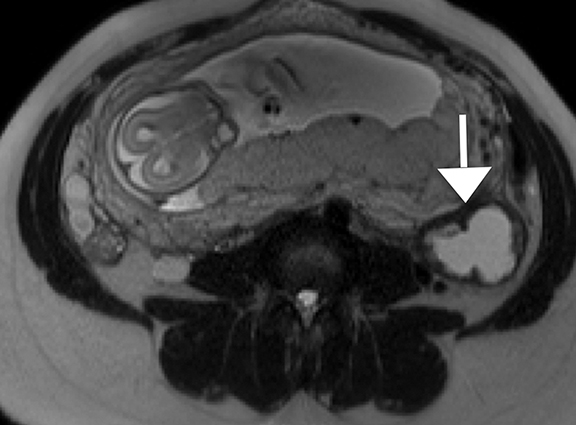
MRI features of obstructive uropathy include an abrupt change in ureteral caliber, renal enlargement, perinephric fluid, and when visible, a low-signal intensity ureteral filling defect on T2-weighted imaging, which reflects the obstructing calculus (Figure 5). Dilatation of the ureter caudal to the sacral promontory also strongly suggests pathologic, rather than physiologic, ureteral dilatation. MRI, however, is limited in the visualization of small calculi in the ureter owing to poor spatial resolution.
Ovarian torsion
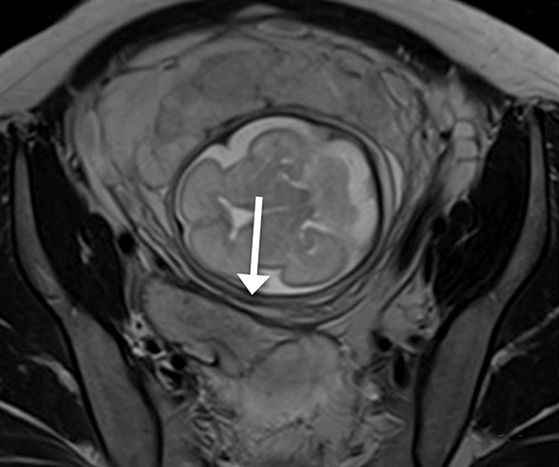
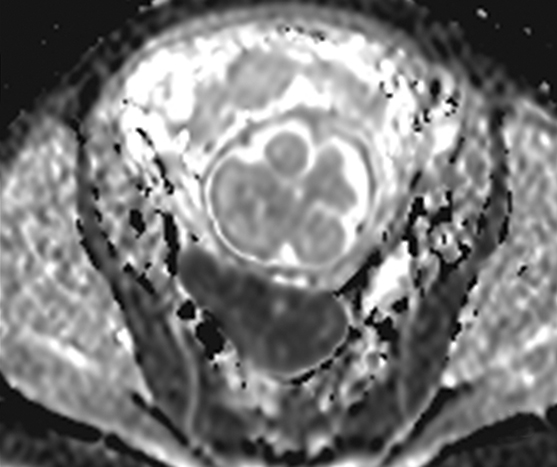
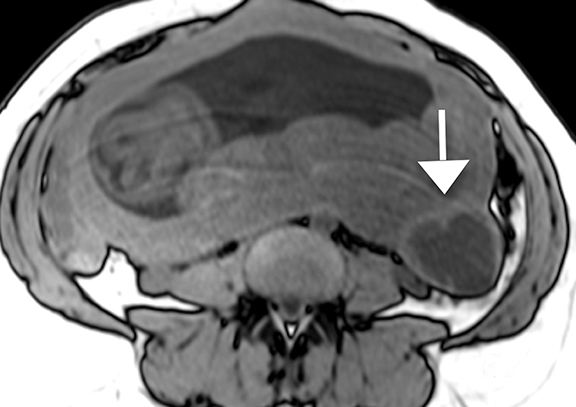
Ovarian torsion occurs when the adnexa twists on its pedicle, leading to vascular compromise. There is a fivefold increase in the rate of ovarian torsion in pregnant women, occurring most frequently in the first trimester.24 While ovarian torsion in pregnancy can occur in a normal ovary due to increased ligamentous laxity, the more common scenario involves an ovarian mass, such as an enlarged corpus luteal cyst, predisposing to torsion.1
MRI features of ovarian torsion include an enlarged ovary, peripheralization of follicles, and hyperintense, edematous, ovarian stroma on T2-weighted imaging. Fallopian tube thickening and a swirled vascular pedicle can also be seen (Figure 6).
Degenerating leiomyoma
The majority of leiomyomas remain asymptomatic during pregnancy. However, as the uterus enlarges in pregnancy, the blood flow to preexisting leiomyomas is altered. When draining veins become obstructed, hemorrhagic infarction or so called red degeneration ensues. This occurs in approximately 8% of leiomyomas during pregnancy and can present with an acute abdomen.25
The appearance of red degeneration on MRI is variable depending on the stage of necrosis. On T1-weighted imaging, diffuse high signal intensity is commonly seen and reflects T1 shortening effects of methemoglobin or proteinaceous contents of blood. There may also be an isolated hyperintense rim on T1-weighted imaging surrounding the acutely degenerating leiomyoma, thought to be secondary to blood product confined to thrombosed vessels.25,26 T2-weighted sequences demonstrate variable signal intensity depending on the age of blood products (Figure 7).
Conclusion
Abdominal pain in pregnancy can be a diagnostic challenge for clinicians and MRI can serve as a critical imaging tool. MRI is considered a safe imaging modality in pregnancy at any gestational age. The benefits of MRI in pregnancy include multiorgan system evaluation without the cost of ionizing radiation. Utilizing a standardized MRI approach, many common gastrointestinal and genitourinary causes of abdominal pain in pregnancy, such as those reviewed in this article, can be accurately diagnosed.
References
- Spalluto LB, Woodfield CA, DeBenedictis CM, et al. MR imaging evaluation of abdominal pain during pregnancy: Appendicitis and other nonobstetric causes. Radiographics. 2012;32(2):317-334.
- Cappell MS, Friedel D. Abdominal pain during pregnancy. Gastroenterol Clin North Am. 2003;32(1):1-58.
- Pedrosa I, Levine D, Eyvazzadeh AD, et al. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3):891-899.
- Kanal E, Barkovich J, Bell C, et al. ACR guidance document on MR safe practices:2013. J Magn Reson Imaging. 2013;37(3): 501-530.
- Tremblay E, Thérasse E, Thomassin-Naggara I, et al. Guidelines for the use of medical imaging during pregnancy and lactation. Radiographics. 2012;32(3):897-911.
- Webb JA, Thomsen HS, Morcos SK, et al. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005;15(6):1234-1240.
- Bendeck SE, Nino-Murcia M, Berry GJ, et al. Imaging for suspected appendicitis: negative appendectomy and perforation rates. Radiology. 2002;225(1):131-136.
- Woodfield CA, Lazarus E, Chen KC, et al. Abdominal pain in pregnancy: Diagnosis and imaging unique to pregnancy—Review. AJR Am J Roentgenol. 2010;194(6 Suppl):14-30.
- Cobbon LP, Groot I, Haans L, et al. MRI for clinically suspected appendicitis during pregnancy. AJR Am J Roentgenol. 2004;183(3):671-675.
- Lee KS, Rofsky NM, Pedrosa I, et al. Localization of the appendix at MR imaging during pregnancy: utility of the cecal tilt angle. Radiology. 2008;249(1):134-141.
- Leeuwenburgh MM, Wiarda BM, Bipat S, et al. Acute appendicitis on abdominal MR images: training readers to improve diagnostic accuracy. Radiology. 2012;264(2):455-463.
- Perdue PW, Johnson HW, Stafford PW. Intestinal obstruction complicating pregnancy. Am J Surg. 1992;164(4):384-388.
- Stukan M, Kruszewski WJ, Dudziak M, et al. Intestinal obstruction in pregnancy. Ginekol Pol. 2013; 84(2):137-141.
- Unal A, Sayharman SE, Ozel L, et al. Acute abdomen in pregnancy requiring surgical management: A 20-case series. Eur J Obstet Gynecol Repro Biol. 2011;159(1):87-90.
- Vassiliou I, Tympa A, Derpapas M, et al. Small bowel ischemia due to jejunum volvulus in pregnancy: a case report. Case Rep Obstet Gynecol. 2012;2012:1-2.
- Gaikwad A, Ghongade D, Kittad P. Fatal midgut volvulus: a rare cause of gestational intestinal obstruction. Abdom Imaging. 2010;35:(3)288-290.
- McKenna DA, Meehan CP, Alhajeri AN, et al. The use of MRI to demonstrate small bowel obstruction during pregnancy. Br J Radiol. 2007;80(949):e4-11.
- Martin DR, Danrad R, Herrmann K, et al. Magnetic resonance imaging of the gastrointestinal tract. Top Magn Reson Imaging. 2005;16(1):77-98.
- Semins MJ, Matlaga BR. Management of urolithiasis in pregnancy. Int J Womens Health. 2013;5:599-604.
- Horowitz E, Schmidt JD. Renal calculi in pregnancy. Clin Obstet Gynecol. 1985;28(2):324-338.
- Puskar D, Balagović I, Filipović A, et al. Symptomatic physiologic hydronephrosis in pregnancy: incidence, complications, and treatment. Eur Urol. 2001;39(3)260-263.
- Mayer IE, Hussain H. Abdominal pain during pregnancy. Gastrienterol Clin North Am. 1998;27(1):1-36.
- Spencer JA, Chahal R, Kelly A, et al. Evaluation of painful hydronephrosis in pregnancy: Magnetic resonance urographic patterns in physiological dilation versus calculous obstruction. J Urol. 2004;171(1):256-260.
- Masselli G,Brunelli R, Monti R, et al. Imaging for acute pelvic pain in pregnancy. Insights Imaging. 2014;5(2):165-181.
- Kawakami S, Togashi K, Konishi I, et al. Red degeneration of uterine leiomyoma: MR appearance. J Comput Assist Tomogr. 1994;18(6):925-928.
- Ueda H, Togashi J, Konishi I, et al. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiographics. 1999;19(1):131-145.
Citation
JA S, KS L. MRI in pregnancy: Gastrointestinal and genitourinary pathology. Appl Radiol. 2017;(5):23-28.
May 10, 2017