Multidetector CT angiography of the carotid arteries




Dr. Rastogi is a Resident Physician and Dr. Villablanca is an Associate Professor, Department of Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA.
Computed tomography angiography (CTA) is a valuable tool for the evaluation of carotid artery disease. It has been used to detect early atherosclerotic changes within the carotid arteries, determine stenosis severity, identify the distribution and extent of atherosclerotic plaque, perform plaque characterization, and detect and characterize carotid dissections. Recent investigations have also explored its role after carotid stenting.
Technique
Successful multidetector CTA (MDCTA) of the carotid arteries is dependent on high image quality. This is achieved through the use of optimized image acquisition and postprocessing protocols. Specific imaging parameters vary by scanner type and number of detector arrays. Generic factors include: 120 kVp, 280 to 400 mA, 18-cm field of view (FOV), 512 × 512 matrix, 0.5- to 1-mm slice collimation, low pitch factor, and a 50% reconstruction overlap. This permits isotropic voxel sizes in the range of 0.5 mm. Isotropic scanning with small voxel size is desirable for optimized 3-dimensional (3D) viewing. Scan coverage is generally from the level of the aortic arch to the skull base. Intravenous (IV) contrast is delivered via a 22-gauge or larger angiocatheter positioned within the right antecubital vein, injecting a nonionic contrast agent with a concentration of 350 mmol/L or higher at a rate of 3 to 4 mL/sec. A right-sided injection will minimize image degradation from beam-hardening artifacts arising from high-density contrast in the central venous structures. 1
Optimized postprocessing and display techniques are as important to high image quality as the scanning parameters. Before selecting a postprocessing workstation, you should ensure that it meets your needs for image quality, processing speed, connectivity, and remote viewing capabilities. Perspective volume rendering (VR) or thick-slab maximum-intensity projection (MIP) are popular techniques for general 3D viewing. However, for precise measurements and stenosis quantification, only the 2-dimensional (2D) gray-scale axial or curved oblique multiplanar reformat (MPR) images should be used. This is because MDCTA axial or axial oblique source images have been shown to correlate more closely with digital subtraction angiography (DSA) than do either MIP or surface-shaded display (SSD) images for all degrees of stenosis. 2 On the 2D gray-scale images, measurements are made using a visual estimation of the full width at half maximum of the slice sensitivity profile at the vessel edge. Measurement errors can be minimized by using the appropriate window and level settings, approximately 1000 W/500 L for 2D gray-scale images. When 3D rendering techniques are used for image review, VR has been shown to be superior to SSD and MIP images.Approximate recommended window/level settings for 3D image viewing are 250 W/150 L, with fine adjustment as needed, to just reach mild background speckling. 3
Carotid artery intima-medial thickness
Clinically significant carotid artery stenosis is a late finding in the progression of atherosclerotic disease. It is now possible to intervene early with aggressive medical therapy to delay or potentially reverse the progression of athero- sclerotic plaque. 4 Carotid artery intima-medial thickness (IMT) has been reported to be a marker of subclinical atherosclerosis 5 and a strong predictor of subsequent cardiovascular complications. 6 Intima-medial thickness consists of both an intimal atherosclerotic process and medial hypertrophy, and it has been well characterized by B-mode ultrasound (US). 7 Intima-medial thickness may also provide a comprehensive view of the consequences of multiple risk factors over time on the arterial wall, including 1) traditional risk factors, 2) emerging risk factors (eg, lipoproteins, plasma viscosity and hyperhomocysteinemia), and 3) various measures of end-organ damage. 7
The use of MDCTA source images in the visualization of carotid wall thickening has not been well explored. Preliminary results with the use of MDCTA show it may be of value in this application by detecting circumferential thickening in the wall of the distal common carotid artery in the absence of overt hypodense atheromatous plaque. 8 The information is easily extracted from CTA data sets and may be routinely reported. Methodologic standardization and validation of measurements still need to be implemented for both US and CTA before routine measurement of IMT can be used as a diagnostic tool in cardiovascular risk stratification, for primary prevention, and in the decision-making process prior to interventional therapy. A value of IMT by US of approximately 0.68 mm has been reported to correlate with an increased risk of atherosclerotic disease. 9 However, IMT varies with age and gender and will likely require interpretation using age and sex-adjusted mean values. 10
Carotid artery stenosis
Stroke is the third leading cause of death in developed countries and is one of the leading causes of adult disability. 11 Approximately 44% of all ischemic strokes are attributed to carotid artery disease. 12 The North American Symptomatic Carotid Endarterectomy Trial (NASCET) 13 and European Carotid Artery Surgery Trial (ECAS) 14 studies established the benefits of carotid end-arterectomy (CEA) in symptomatic patients with stenosis >60%. 15 In the NASCET study, the cumulative risk of any ipsilateral stroke was found to be 26% at 2 years in the aspirin-treated patients, and 9% in the surgically treated patients, with a 30-day perioperative risk of any stroke or death of 5.8%. In pa-tients with <60% carotid stenosis, the risk of surgery does not appear to be justified. 13 The benefits of CEA for asymptomatic patients with >70% stenosis have been reported by the international Asymptomatic Carotid Surgery Trial (ACST), which found a 6% absolute risk reduction and a 50% relative risk reduction of stroke over 5 years in the treated group. 16 This underscores the importance of accurate and reproducible measurements of carotid stenosis when stenosis criteria are used for patient selection. 17
The gold standard for the detection and grading of carotid stenosis has been catheter angiography. 13 However, Willinsky et al 18 reported angiography of the carotid arteries carried a 1.3% risk of neurologic complication, 0.5% of which can be permanent. In the Asymptomatic Carotid Atherosclerosis Study, nearly half of the morbidity/mortality rate (2.6%) was accounted for by the rate of stroke after DSA (1.2%). 19 A desire for enhanced patient safety and lower study costs has prompted the development of noninvasive tools using duplex US, magnetic resonance imaging (MRI), and CT. All of these noninvasive techniques appear to be suitable for measuring stenosis of the proximal internal carotid arteries when compared with DSA as determined by a meta-analysis of studies between 1990 to 2001. 20 All of these techniques also show similar accuracy in the diagnosis of symptomatic carotid stenosis. However, each of these imaging techniques has its own advantages and limitations.
Carotid US is a noninvasive, low-cost, and widely available modality, but it suffers from significant drawbacks. It is operator-dependent, and findings can be variable and difficult to reproduce. 21,22 Lee et al 23 showed that the variability of Doppler measurements in the common carotid artery (CCA) and internal carotid artery (ICA) in 85 patients without carotid disease is substantial and could lead to inaccuracies in carotid arterial stenosis measurements. They report that peak systolic velocity ratios exceeded 1.8 (suggesting ≥60% stenosis) in 14% of normal carotid arteries and that end diastolic velocity ratios exceeded a ratio of 2.4 (also suggesting >60% stenosis) in 15% of normal carotids. Despite its common usage, no universal table of measurement standards that reliably estimates the degree of stenosis has been established. Variability in velocity measurements along the course of the ICA also influences the interpretation of ratio parameters. 23 Peak systolic velocity has been shown to be influenced by relative position within the ICA, equipment type, technique used, and by physiologic factors, such as cardiac output and contralateral restriction of blood flow. 24 Another limitation of US is its limited FOV that renders the evaluation of tandem stenoses and vessel course difficult. The latter is particularly important, as kinked carotid arteries are better suited for CEA than for carotid stenting. Lastly, heavy calcific plaque, a common finding in atherosclerotic disease of the carotid bifurcation, works as an acoustic curtain through which insonation is impaired. If the point of maximal luminal narrowing occurs within the calcified segment, forcing a sampling distal to the site of maximal peak systolic velocity will lead to an under-estimate of the percent stenosis.
MR angiography (MRA) of the carotid arteries has undergone significant technologic improvements during the past several years. Many of the initial studies that investigated the utility of MRI for carotid stenosis were based on time-of-flight (TOF) techniques, which have significant limitations. 25,26 Since TOF-MRA is a flow-dependent technique, slow and/or turbulent flow patterns, as are commonly encountered at sites of significant atherosclerotic luminal narrowing, can lead to spin dephasing and resultant signal loss. As a result, TOF-MRA frequently overestimates carotid stenosis severity. 27 More recently, significant improvements in MRA have been achieved through the development of contrast-enhanced MRA techniques, which are less susceptible to the artifacts associated with TOF-MRA techniques. However, in comparison to MDCTA, both clinical TOF-MRA and contrast-enhanced MRA at 1.5T currently have a lower spatial resolution. 28 This may be important, since accurate submillimeter measurements of carotid stenosis diameters may make the difference between a clinically significant and a nonsignificant lesion based on NASCET criteria. Recent work suggests that contrast-enhanced MRA at 3T provides superior image quality to both TOF-MRA and contrast-enhanced MRA at 1.5T, approximating the spatial resolution of MDCTA. 29
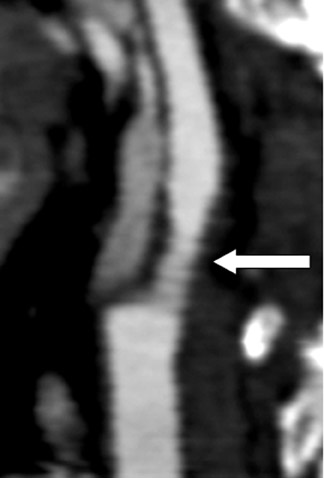
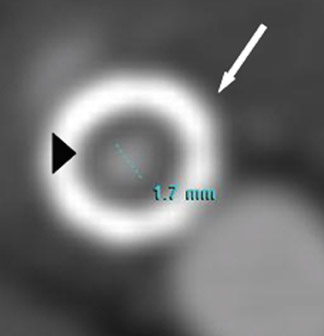
Multidetector CTA of the carotid arteries has been shown to be a suitable noninvasive technique for assessing carotid artery stenoses. 2,30-32 A meta-analysis of studies from 1990 to 2003 showed that CTA had a pooled sensitivity and specificity of 85% and 93%, respectively, for the detection of 70% to 99% stenoses. 33 Others report that patients tend to prefer CTA over MRA or DSA, 34 with US preferred by patients overall. In specific instances, MDCTA can also outperform DSA in the accurate depiction of carotid stenosis. With DSA, the standard of care is 2 or 3-view carotid angiograms, generally in the anteroposterior, lateral, and, occasionally, right or left posterior oblique projections. When a stenosis is markedly eccentric or elliptical and when the long axis of that ellipse is not along the beam path of these standard projections, the stenosis severity will be underestimated by DSA. This measurement error can be on the order of ≥25%, and could erroneously exclude an otherwise qualified candidate from CEA. The problem is not easily solved, as it is not possible for the angiographer to intuit a priori which precise projection will render the lumen in its narrowest profile. Hirai et al 35 recently showed that when the carotid artery lumen has a high long-to-short axis ratio, the luminal morphology may lead to erroneously decreased estimates of luminal stenosis by DSA (Figure 1).
A possible limitation of CTA is the difficulty of measuring stenoses in a heavily calcified vessel. 25,36 However, numerous investigators have noted that with appropriate window and level adjustments, the blooming artifact from the heavy calcific plaque can be reduced, allowing for a more accurate quantification. 28,37 The major disadvantages of CTA compared with MRA include its use of ionizing radiation and potential for nephrotoxicity.
How to measure carotid artery stenoses
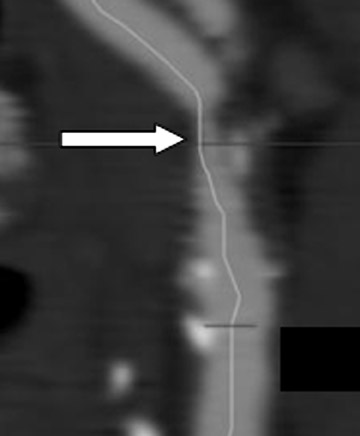
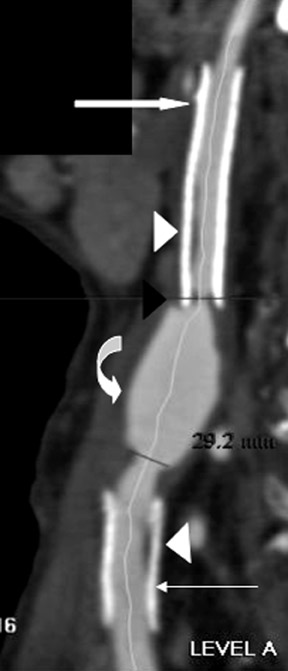
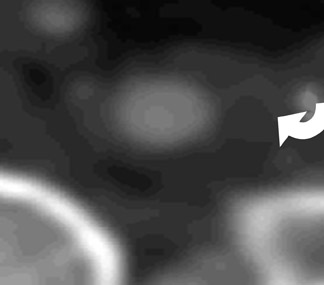
The NASCET criteria require measurement of the stenotic lesion at the site of maximal luminal narrowing and in the first normal distal arterial segment where the walls are parallel. 13,14,17 With the ECAS method, the stenosis is calculated by measuring the ratio between the narrowed carotid bulb luminal diameter and the native carotid bulb from wall to wall. Both methods can be reliably performed and reported using MDCTA. The axial or axial oblique CTA source images provide the most reliable stenosis measurements when comparing CTA with DSA. 2 In cases in which the vessel is tortuous, it is important to use the curved MPR to ensure true cross-sectional measurement perpendicular to the long axis of the vessel, and thus avoid artifactual luminal eccentricity (Figure 2). Appropriate window and leveling is also crucial when measuring carotid stenosis, especially when significant atherosclerotic calcification is present. For the interpretation of axial or axial oblique source data, the authors recommend using approximate window and level settings of 1000 W/ 500 L. In an effort to simplify the measurement of carotid stenoses by CTA, Bartlett et al 28 have recently proposed the direct measurement of carotid bulb diameter and its correlation to the percent stenoses as determined by the NASCET criteria. According to their study, a carotid bulb diameter >2.2 mm corresponds to a <50% carotid stenosis, and a carotid bulb diameter <1.3 mm corresponds to a <70% stenosis.
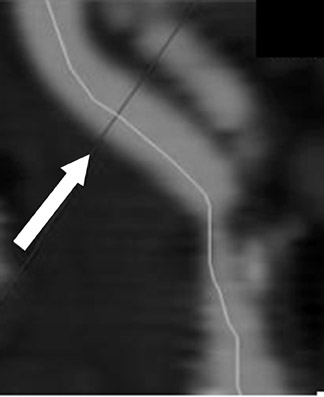
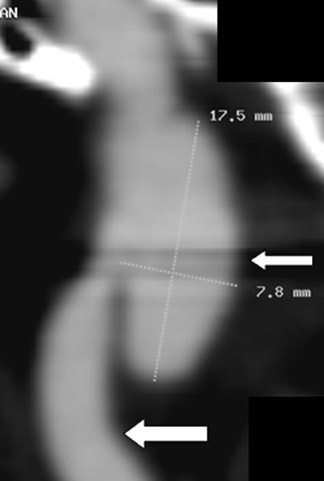
The measurement of near occlusion deserves special attention because of its clinical relevance and its determination using CTA. In patients with near-total occlusion, the benefit of CEA over medical therapy has been shown to be clinically significant but far less profound than that seen for patients with severe carotid artery stenoses (stenoses >70%). 15,38 In determining chronic near-total occlusion, the traditional measurement of carotid stenoses may lead to an underestimation of the stenoses because of the associated loss of diameter of the ipsilateral ICA distal to the stenosis (CTA string sign). 39 Near-total occlusion at CTA may be best determined by noting a signifi-cant carotid stenosis with an associated reduction in ipsilateral ICA to contralateral ICA diameter (>80%) and reduction in ipsilateral ICA diameter to the ipsilateral external carotid artery (ECA) diameter (ICA/ECA ratio <1.25). 28,39 CT angiography is believed to be comparable to DSA in the determination of near-total occlusion and to be superior to both US and MRA in this setting, as those modalities commonly suggest occlusion. The distinction between carotid artery occlusion and near occlusion can also be made reliably with CTA, which has a reported sensitivity and specificity of 97% and 99%, respectively, for carotid artery occlusion 33 (Figure 3). This is clinically important because the therapeutic alternatives available to patients with near occlusion include revascularization, whereas patients with established occlusions will not benefit from CEA but may benefit from extracranial to intracranial bypass, medical management, and/or contralateral CEA. 40
Carotid plaque characterization
Although the risk of stroke has been most closely associated with the severity of carotid stenosis, 13-15 atheroembolic strokes are known to occur in patients with less than severe (<70%) stenoses, and conversely, not to occur in patients with known severe stenoses. 41 These findings suggest that carotid plaque morphology may play an important role in the risk of atheroembolic stroke. Certain plaque morphologies have been associated with higher stroke risk. Histologic characteristics associated with increased stroke risk include a large lipid/necrotic core, a thin or ruptured fibrous cap, plaque ulceration, intraplaque hemorrhage, and a dense inflammatory cellular infiltrate. 42,43 Preliminary studies suggest that CTA is able to provide accurate plaque characterization when compared with histopatho-logic specimens. 31,44 Through the use of Hounsfield unit (HU) analysis, CT allows for the potential characterization of plaque composition, specifically commenting on the lipid/necrotic core, fi-brous component, and calcification. Areas of the plaque that have HU similar to muscle have been proposed to likely correspond to fibrous tissue, whereas areas with low attenuation values are thought to represent the lipid/necrotic core. 31,44 A more recent study, however, questions the ability of CTA to characterize plaque morphology. 45 This suggests that additional studies are needed to determine the true capabilities of MDCTA in plaque characterization, particularly with respect to the identification of vulnerable or unstable plaque elements.
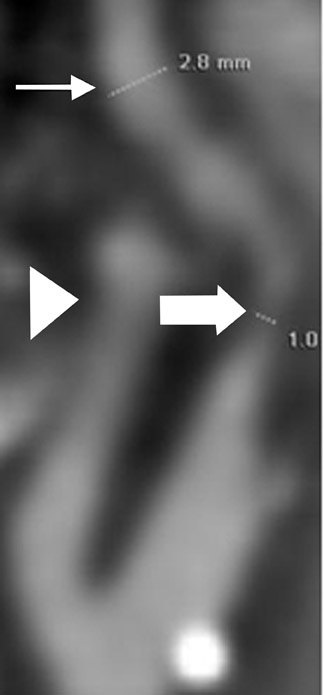
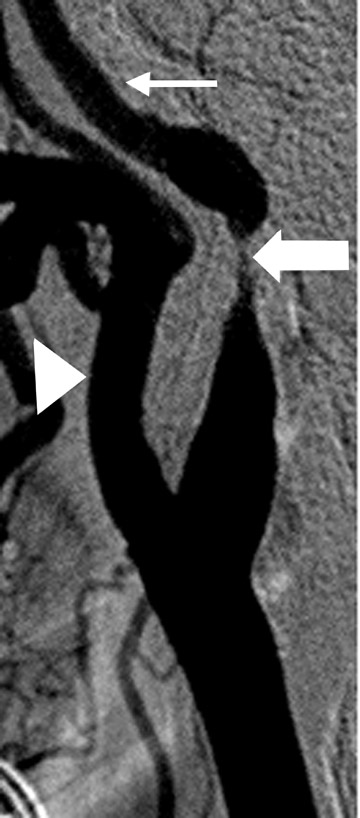
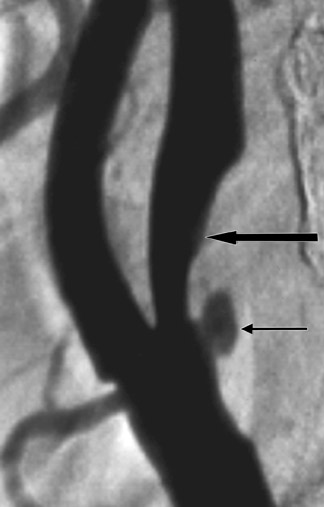
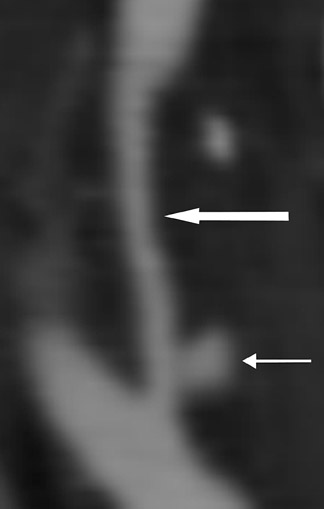
Ulcerations, a known cause of thromboembolic phenomena from carotid atherosclerosis independent of stenosis severity, can be missed on DSA when the ulcer crater projects along the path of the X-ray beam. 46 Multidetector CTA is capable of excellent depiction of plaque ulcerations and arguably outperforms DSA in crater detection. 47 Because MDCTA is a direct contrast cast of the vessel lumen, it is generally superior to both TOF-MRA and US in the detection of ulcer craters (Figure 4).
Evaluation of carotid stents
Recently, endovascular treatment of clinically or hemodynamically significant carotid artery stenoses has gained in popularity. Currently, carotid stenting is approved only for high-risk surgical patients. However, multiple trials are under way to establish the equivalence or superiority of carotid stenting with CEA. 48 In comparison to CEA, carotid stenting is thought to be associated with higher rates of restenoses, up to 18%. 49 Most patients are currently evaluated with duplex US yearly and are referred for DSA when findings are suggestive of high-grade stenosis. However, duplex US of poststent carotid arteries has shown a high false-positive rate of >30%. A preliminary study by Orbach et al 50 has shown that MDCTA may be useful in the evaluation of the poststent carotid artery. Multidetector CTA reveals the location, size, posture, and patency of arterial stents. Most importantly, their results suggest CTA has reasonable correlation with DSA when appropriate window and leveling was used, and that MDCTA is promising for the detection of intrastent stenoses from recurrent atherosclerosis and intimal hyperplasia (Figure 5). In their methodology, they recommend using a window width of 1500 HU and level of 400 HU, notably wider than the typical window widths used for MDCTA, in order to decrease the blooming artifact associated with the stent. Orbach et al, 50 working with a phantom model, advise the use of a kernel optimized for high- attenuation sharp edges, as an acquisition tool for improved detection of intra-stent stenoses. 50
Carotid artery dissection
Dissection of the extracranial ICA is responsible for up to 10% to 25% of ischemic stroke in young and middle-aged patients. 51 The ICA is the most commonly involved cervical vessel. 52 Cerebral ischemia may result from hemodynamic compromise or embolic complications. The average annual incidence of carotid artery dissection (CAD) ranges from 2.5 to 3 per 100,000. 52 The pathogenesis of CAD remains unknown in the majority of cases. Trauma and primary diseases of the arterial wall are the principal predisposing factors. Clinical presentation during the acute phase may include headache, ipsilateral neck pain, and Horner's syndrome. A smaller percentage may be silent. 53
Angiography has been the gold standard for evaluating the presence and extent of arterial dissection. 54 Typical angiographic findings for nonocclusive dissection include an elongated or tapered stenosis (rat-tail sign) with or without the presence of a pseudoaneurysm. 55 Because DSA does not visualize the arterial wall, it can lack specificity in occlusive dissection, which makes it it difficult for DSA to differentiate between occlusive dissection and other causes of arterial occlusion, including thromboembolism and atherosclerotic disease. 56
Prior work has shown that both Doppler US and MRI may be of value in the diagnosis of cervical carotid artery dissection. 57 Both techniques have significant limitations, however. The primary limitations of Doppler US are a limited scan coverage using conventional probes and the nonspecific and overlapping nature of the echo patterns obtained from dissected arteries.
The limitations of TOF-MRA include artifacts arising from the loss of signal intensity derived from the loss of proton spin coherence in the setting of reduced luminal diameter. Additional limitations include a potentially difficult diagnosis in the setting of fresh hematoma (not T1-weighted [T1W] hyperintense) and nonspecific imaging when high signal covers all of the section of the artery, leading to confusion between mural hematoma and possible thrombus in the true lumen. 58 Also, the use of MRI for CAD is limited in the setting of acute trauma since it is incompatible with life support equipment, provides poor evaluation of associated osseous injuries, and requires prolonged scan times. Nevertheless, MRI constitutes a valuable tool for detecting cervical dissection. Typically, axial T1W images show hyperintense crescentic wall thickening surrounding a narrowed signal void-the lumen. The technique may also be used in the setting of occlusive dissection by the use of the axial T1W images. 59 Recent reports suggest that first-pass gadolinium-enhanced MRA may be superior to TOF-MRA. 60,61
Helical CTA has also been shown to be a reliable method for evaluating extracranial ICA dissection, especially in the setting of trauma. 62 Leclerc et al, 63 in a study of 18 patients, found a 100% sensitivity and 100% specificity for both stenotic and occlusive dissections. They found an analysis of the residual arterial lumen and the measurement of the external diameter of the carotid artery to be the most sensitive criteria for the diagnosis of CAD. Our experience shows that criteria more specific than luminal reduction and external diameter can also be used to establish the diagnosis of CAD. These include the presence of an intimal flap, the presence of an isodense eccentric or concentric intramural hematoma, formation of dissecting aneurysms, increased native arterial diameter, and luminal stenosis (Figure 6). It is our opinion that because MDCTA provides non-luminal information, the specificity of MDCTA for carotid dissection may in some instances be higher than that of DSA for carotid dissection when typical angiographic findings are lacking or ambiguous. In 1996, Egelhof et al, 64 in a study population of 21 patients with clinically or sonographically suspected extra-cranial carotid dissection, reported a 100% sensitivity and specificity of CTA as compared with catheter angiography. At our institution, we have found that direct MDCTA signs of arterial dissection do not require further diagnostic investigation to confirm the diagnosis. Specific CTA findings include an intimal flap, dissecting aneurysms, and mural hematomas, as described below.
An intimal flap is a thin, hypodense band that spans the arterial lumen; it is generally oblique or spiral in nature and is of high sensitivity. Very thin web stenoses and horizontally oriented intimal shelves may be difficult to detect by MDCTA.
Dissecting aneurysms appear as focal contrast-opacified outpouchings arising from the parent artery. These lesions may contain intraluminal thrombus and may exert mass effect on the parent artery, which contributes to luminal reduction of the parent vessel. The sensitivity of CTA for dissecting aneurysms is very high (Figure 4).
Mural hematomas can be iso- or hypodense to surrounding tissue, depending on whether they are acute or subacute in nature. The adventitia of the parent artery is clearly visualized peripheral to the hematoma. The hematoma may be focal or segmental, concentric or eccentric. Using appropriate window and level settings, the detection of a mural hematoma is straightforward when using gray-scale 2D source or MPR images.
Typically, patients with CAD are treated with anticoagulation for 3 to 6 months after the initial event to prevent thromboembolic events. 50 Approximately 70% of dissections heal spontaneously, and most pseudoaneurysms either resolve or significantly decrease in size. Only ≤15% of cases of CAD are noted to progress. 64 Multidetector CTA can be used to detect progression and document healing.
Artifacts and pitfalls
Several artifacts and pitfalls have been associated with MDCTA in the evaluation of carotid pathology. Swallowing artifacts appear as focal blurring within the source data and may lead to an inability to obtain accurate quantification. Beam-hardening artifacts arise from dense objects (such as dental amalgam, vascular clips, or very dense calcifications) and may mimic arterial stenosis or dissection (Figure 5). 66
Sources of measurement errors include a failure to use optimal window-level setting, stenosis measurements using MPR images nonorthogonal to the long axis of the vessel of interest, suboptimal contrast opacification, and the use of images containing gross patient motion. Failure to cover the entire region of interest may lead to incomplete visualization or characterization of relevant pathology or to false-negative findings. All studies should use automatic bolus detection technology to trigger helical contrast acquisition. Alternately, a timing injection can be performed in all patients.
Overlapping venous and arterial anatomy may obscure pathology on VR images. This may be avoided by the use of bolus-detection techniques and by the use of 2D image data for all detection and quantification. Beam-hardening artifacts that arise from shoulder bones, clavicles, and hyperconcentrated contrast material arriving via the brachiocephalic and subclavian veins may degrade image quality in the lower neck. Injection of contrast into the right upper extremity may help to minimize these artifacts. 1
Conclusion
Multidetector CTA is emerging as a versatile and powerful tool in the noninvasive evaluation of a range of pathologies affecting the extra- and intracranial segments of the carotid arteries. Optimized scan and image acquisition protocols, high-quality image postprocessing, and a comprehensive understanding of the appearance of common pathologies and imaging artifacts will help ensure accurate lesion detection, quantification, and characterization.
