Neuroendocrine tumor of the breast
Images


CASE SUMMARY
A 39-year-old G2P1 female presented with a new palpable right breast lump, which she had noticed while breastfeeding. Her risks for breast cancer included a maternal grandmother who was diagnosed with breast cancer in her 50s and late childbearing at age 37. On physical examination, she had a large palpable non-tender mass in the lower right breast.
IMAGING FINDINGS
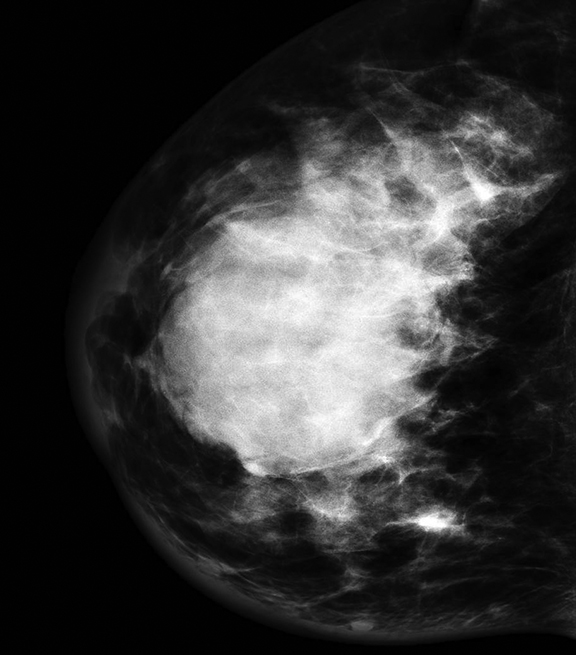
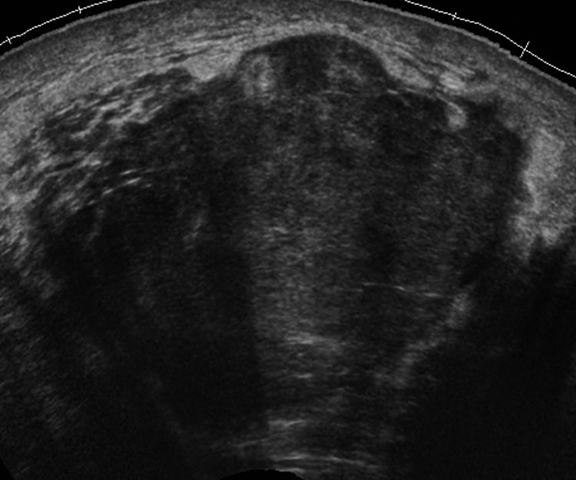
Ultrasound of the palpable mass in the right breast revealed a 4.5 × 4.8 × 4.1 cm circumscribed hypoechoic mass at the 6:00 location. The mass was felt to represent a lactating adenoma. An ultrasound-guided biopsy was recommended. The patient presented one month later for the biopsy. The mass had increased to 6 cm at the time of the biopsy. Mammogram revealed a 6 cm round mass with partially circumscribed margins in the central right breast. There was also skin thickening noted in the inferior medial right breast on the mammogram.
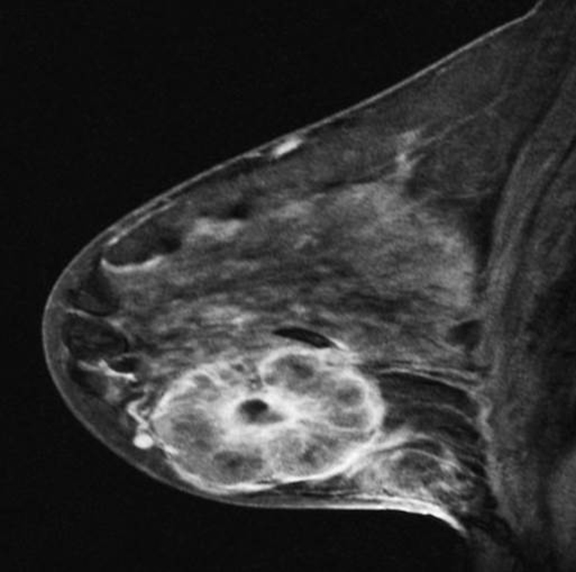
Ultrasound-guided biopsy of the mass was performed. MRI breast showed a large 6 cm rim enhancing right breast mass. Also, diffuse skin thickening and edema was noted in the right breast. There were multiple enlarged and necrotic right axillary lymph nodes, as well as right internal mammary lymphadenopathy.
Fine-needle-aspiration of a right axillary lymph node was positive for malignancy.
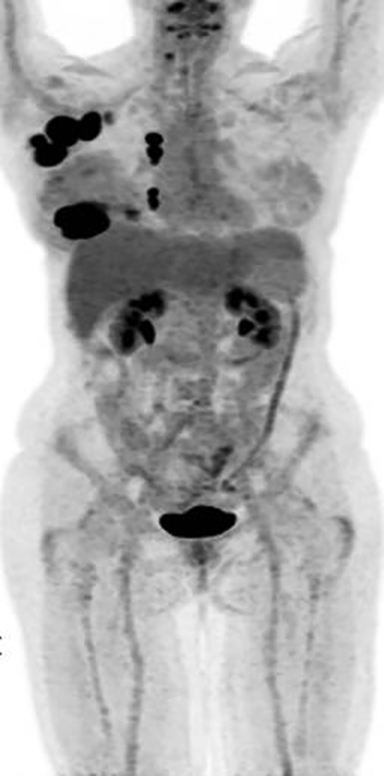
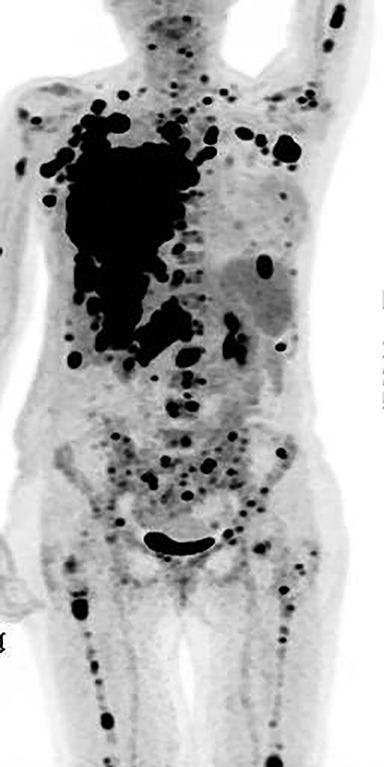
Metastatic workup including a bone scan, a CT chest/abdomen/pelvis and MRI brain were negative for metastatic disease or evidence of any other primary malignancy elsewhere in the body.
Ultrasound-guided biopsy of the right breast mass revealed high-grade neuroendocrine tumor with small cell features; ER/PR negative, Her-2 negative and Ki-67 positive at 99%. The tumor cells were positive for CD56 and synaptophysin, AE1/AE3 and CAM 5 and negative for chromogranin.
DIAGNOSIS
Neuroendocrine tumor of the breast
DISCUSSION
Primary neuroendocrine cancer (NEC) of the breast is very rare with a limited number of cases reported in the literature. The first case of this type of tumor in the breast was reported by Wade, et al, in 1983.1 Small cell carcinoma of the lung is well known, but can also occur in many extra pulmonary sites, such as bowel, pancreas, bladder, salivary glands, skin, breast, cervix and prostate. Primary small cell carcinoma in the breast cannot be differentiated from ones arising from any other site based on the histology and immunohistochemistry. Therefore, exclusion of an extra mammary primary site and/or demonstration of an in situ component within the breast should be confirmed for the diagnosis of primary neuroendocrine carcinoma of the breast.2 The differentiation between primary and metastatic tumor is essential for treatment planning.
The histologic characteristics of primary small cell neuroendocrine carcinomas are cellular monotony, fine granular (salt and pepper) chromatin, inconspicuous nucleoli, pseudorosettes and palisades and a high mitotic rate.3-5 However, these features are inconsistently seen and pathologists rely on a panel of neuroendocrine markers. In the WHO classification, neuroendocrine tumors have been defined as those in which one or more neuroendocrine markers, such as neuro specific enolase, chromogranin A, and/or synaptophysin, are expressed in at least 50% of cancer cells. In the WHO classification of breast tumors, these tumors have been classified as: solid neuroendocrine carcinoma, atypical carcinoid tumor, small cell/oat cell carcinoma and large cell neuroendocrine carcinoma.6
The imaging features of neuroendocrine breast tumor are nonspecific but differ from the typical invasive breast cancer. In one of the largest studies on 87 cases of primary NEC of the breast, the most common mammographic presentation of this malignancy was a round or oval high-density mass with nonspiculated margins.6 On ultrasound, the most common feature was a solid hypoechoic, irregular and hypervascular mass with either posterior acoustic enhancement or no posterior acoustic features. Majority of tumors in this series were ER positive and HER2/neu negative, although the receptor status has been shown to be variable in some other studies. These tumors are usually more circumscribed and large at presentation, compared to other invasive carcinomas in the breast and often have lymph node metastasis at the time of diagnosis.7, 8
The age of incidence is usually between 40-70 years. The majority of patients present with a firm palpable mass. The size of the tumor can range from 1 cm to 15 cm (median 3.5 cm). The prognosis depends on the size at initial presentation and presence or absence of lymph node metastasis.9 It also depends on the grade of the tumor, with the well-differentiated tumors usually having a better outcome than the poorly differentiated tumors.3 The prognosis is usually poor, similar to small cell carcinoma of the lung.
Due to the small number of cases, no standard treatment protocol has been established for this type of tumor in the breast. Modified radical mastectomy with or without axillary nodal dissection seems to be the treatment of choice followed by adjuvant chemotherapy and possible radiation. The cytotoxic therapy for these tumors is different than invasive ductal/lobular cancer and regimens similar to small cell cancer of the lung have been used. Chemotherapy regimens described in the literature have included FEC (fluorouracil, epirubicin, and cyclophosphamide), CMF (cyclophosphamide, methotrexate, and fluorouracil), adriamycin and cisplatin, paclitaxel alone, CAE (cyclophosphamide, doxorubicin, etoposide) and CP (cisplatin, etoposide) as single agents or in combinations.3,10
Our patient was started on cisplatin and etoposide chemotherapy and then received 4 cycles of weekly Taxol with minimal clinical and radiographic response.
She had a right mastectomy and right axillary node dissection. The tumor measured 17 cm and had evidence of angiolymphatic invasion, dermal lymphatic invasion, and nipple involvement. Surgical margins were negative. Twenty-one of 26 right axillary lymph nodes showed metastatic disease.
The patient developed cutaneous recurrence of tumor 6 weeks post mastectomy in the right mastectomy bed. Repeat imaging showed rapid disease progression with right pleural, liver, splenic and bony metastases. The patient rapidly deteriorated thereafter and passed away 2 months after the mastectomy.
CONCLUSION
Primary neuroendocrine tumor is a rare, distinct and aggressive malignancy, which comprises about 2% to 5% of the breast cancers using the WHO criteria. Lymph node and distant metastasis is frequent and the prognosis is usually poor, similar to its pulmonary counterpart. Patients may do well, if detected in the early stage of the disease. Further studies are needed in a larger patient population to establish a standard treatment regimen for this rare malignancy.
REFERENCES
- Wade PM, Mills SE, Read M. Small cell neuroendocrine (Oat Cell) carcinoma of the breast. Cancer. 1983;52:121-125.
- Kitakata H, Yasumoto K, Sudo Y, Minato H, Takahashi Y. A case of primary small cell carcinoma of the breast. Breast Cancer. 2007;14:414-419.
- Angarita FA, Rodríguez JL, Meek E, Sánchez JO, Tawil M and Torregrosa L. Locally-advanced primary neuroendocrine carcinoma of the breast: case report and review of the literature. World Journal of Surgical Oncology. 2013;11:128-139.
- Jochems L, Tjalma WAA. Primary small cell neuroendocrine tumour of the breast. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004;115:231-233.
- Zekioglu O, Erhan Y, Ciris M, Bayramoglu H. Neuroendocrine differentiated carcinomas of the breast: a distinct entity. The Breast. 2003;12:251-257.
- Tavassoli FA, Devilee P. Pathology and genetics of tumors of the breast and female genital organs. WHO Classification of Tumors Series. Lyon, France: IARC Press, 2003;32–34.
- Park YM, Wu Y, Wei W, Yang WT .Primary neuroendocrine carcinoma of the breast: Clinical, imaging, and histologic features. AJR. 2014;203:221–230.
- Mariscal A, Balliu E, Díaz R, Casas JD, Gallart AM. Primary oat cell carcinoma of the breast: Imaging features. AJR. 2004;183:1169-1171.
- Yamasaki T, Shimazaki H, Aida S et al. Primary small cell (oat cell) carcinoma of the breast: Report of a case and review of the literature. Pathology International. 2000;50:914-918.
- Latif N, Rosa M, Samian L, Rana F. An unusual case of primary small cell neuroendocrine carcinoma of the breast. The Breast. 2010;16:647-651.
Citation
A W. Neuroendocrine tumor of the breast. Appl Radiol. 2016;(10):27-28.
October 15, 2016