Part 2: MR of the female pelvis
Images



Dr. Agrawal is a Nuclear Medicine Fellow, Division of Nuclear Medicine, Mallinckrodt Institute of Radiology, St. Louis, MO; Dr. Sethi is a Research Associate, Department of Radiology, and Dr. Oto is a Professor, Department of Radiology, Biological Sciences Division, University of Chicago, Chicago, IL.
In this second half of a 2-part series, we discuss recent studies demonstrating the role of dynamic magnetic resonance (MR) imaging and diffusion-weighted imaging in characterizing the benign and malignant process of the endometrium. MR imaging allows accurate distinction between various benign and malignant uterine and ovarian conditions when ultrasound is indeterminate, and can serve as an adjunct to diagnosticlaproscopy, hysteroscopy, hysterosalpingography, and transvaginal USG in patients being evaluated for infertility.
Advanced MR imaging
Recent studies have emphasized the role of dynamic MR imaging and diffusion weighted imaging in characterizing the benign and malignant process of the endometrium. Park et al demonstrated 95% of benign lesions and 100% of sarcomas reach late peak enhancement at 2 to 3 min with persistent strong enhancement on late CE T1WI. On the other hand 72% of endometrial cancers showed early peak enhancement within 1 min and showed weak enhancement with gradual washout on late CE T1WI.27 Wang et al showed that mean ADC of stage 1 endometrial carcinoma was significantly lower than that of normal endometrium and benign endometrial lesions and proposed to add DW imaging as an adjunct in the routine MR protocol for assessment of uterine lesions.28 A non-enhanced MR imaging study by Inada et al demonstrated the mean ADC value of endometrial cancer was significantly lower than those of the normal endometrium, myometrium, leiomyoma and adenomyosis (p<0.05) and concluded that DW imaging can be helpful in the detection of uterine endometrial cancer in nonenhanced MR imaging.29
MR Imaging of the adnexa
Normal adnexal anatomy
The ovaries in patients of reproductive age are more consistently visualized than in postmenopausal women where they may not be seen in up to 60% of patients. On T1WI signal and contains high-intensity follicles while the medulla has intermediate-intensity. During the menstrual phase, the ovaries decrease in size. The ovaries then gradually enlarge during the proliferative phase and during the periovulation phase reach their maximum size and have an enlarged dominant follicle. During this periovulation phase the medulla has high-signal intensity on T2 due to edematous vascularized stroma and may show high signal on DW images likely from T2 shine-through. The fallopian tubes are approximately 10 cm long within the superior portion of broad ligament and normal tubes are not routinely visualized.30-34
Benign masses
The number of benign masses of the ovary far exceeds the number of malignant masses.35 Ovarian masses, such as benign cysts and endometriomas, can be effectively treated with laparoscopic surgery in patients who have nonsuspicious benign imaging features and thus obviate the need of laparotomy in many young patients.36-38 MR imaging is by far the most accurate imaging technique in characterizing an ovarian mass and should be primarily used as problem solving tool serving as an adjunct to ultrasound, which, for practical reasons, remains the initial examination of choice. When utilized appropriately it is a cost effective technique for guiding further management in selected group of patients.39-41
Both T1 (with fat saturation) and T2 acquisition are fundamental. Using a relatively long echo time can accentuate the signal intensity difference in mixed solid cystic lesions. A small field of view, high-resolution matrix, thin sections, and gadolinium-enhanced T1WI improve characterization and detection of ovarian lesions. Benign ovarian cysts: Most ovarian cysts in females of reproductive age are functional cysts.42 Follicular cysts results from failure of the follicle to ovulate or regress. They are generally simple cysts with thin walls (<3mm) and rarely greater than 5 cm. Corpus luteal cyst result from failure of regression of corpus luteum and are usually >2.5 cm. These cysts can be followed up by ultrasound and usually resolve in 2 to 3 cycles.31,43-45 On MRI the cyst wall is generally thicker, has intermediate-signal intensity on T1 and relatively low-signal intensity on T2 and shows enhancement from increased vascularity of the thick luteinized cell layer. These cysts can be complex from hemorrhage and show relatively high-signal intensity on T1 and intermediate to high on T2WI. Hemorrhagic cysts can mimic endometrioma; however, functional cysts lack T2 shortening, a feature of endometriomas from repeated bleed. In case of diagnostic dilemma, follow up with ultrasound can be done for resolution.30,34,44-46
Simple adnexal cysts in postmenopausal females are very common. The prevalence of simple cystic lesion in these women according to2 large ovarian cancer screening trials ranges from 14% to 18%.47,48 Approximately 50% to 70% of cysts resolve on follow up exam and the majority of those that persist remain unchanged.49,50 Risk of malignancy in unilocular ovarian cystic lesion <10 cm in diameter in asymptomatic postmenopausal women is extremely low.51
Endometriosis is a fairly common condition in women of reproductive age group with a prevalence of about 10%. It is characterized by presence of endometrial implants outside of uterine cavity. The most common sites are ovaries, uterine ligament, cul-de-sac, serosal uterine surface, fallopian tubes, rectosigmoid, and bladder dome.
Hemorrhagic products result in T2* effect—diffuse darkening or dependent graded “shading” of fluid contents, bright and dark fluid lev
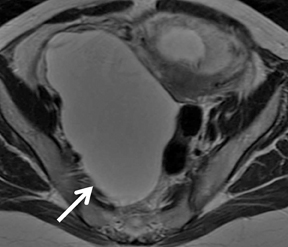
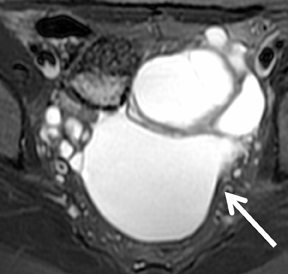
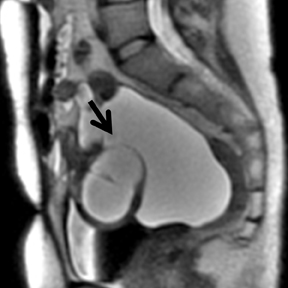
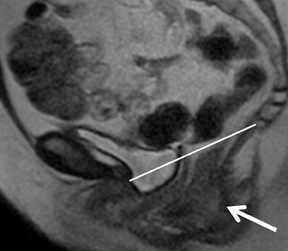
els from sedimentation of blood products. There is usually T2 darkening in the walls due to hemosiderin deposition. Diffuse darkening can be differentiated from fibrous tumors by identifying high T1 signal in endometriomas (Figure 8). Endometriomas acquire an iron concentration many times greater than whole blood resulting in very high-signal intensity similar to fat. Multiplicity is another feature favoring a diagnosis of endometrioma.31,45,52 Sensitivity and specificity of MR imaging vary from 90% to 92% and 91% to 98% respectively.53-57 Malignant transformation occurs in about 1% of cases, usually to endometroid and clear cell carcinoma. In suspicious cases, contrast enhancement can be helpful. Diffuse loss of shading effect on serial exams due to the dilutional effect of secretions from malignant epithelium can be another clue to malignant transformation.58
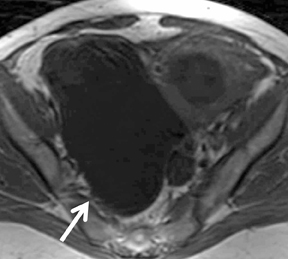
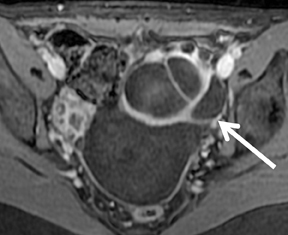
Mature cystic teratoma and dermoid cyst are the most common ovarian neoplasm derived from germ cells constituting approximately20% of all ovarian tumors. These are usually diagnosed on ultrasound and MR imaging may be performed for better characterization of a complex, predominantly solid mass with echogenic foci. Dermoid cysts usually demonstrate visible fat as high-signal intensity on both T1and FSE T2-weighted images. Out-of-phase imaging (selective fat suppression technique) has a sensitivity of 92% to 95% for differentiation from hemorrhagic adnexal cyst. Focal T2 darkening from calcifications, ossification or dental elements can be seen (Figure 9). Once the tumor is characterized, contrast-enhanced images are usually not required.59,60 Malignant transformation occurs in about 2% of mature teratomas. Invasive squamous cell carcinomas arising from skin elements are the most common type and predominantly affect postmenopausal women. The tumor spreads transmurally and invades adjacent organs.61
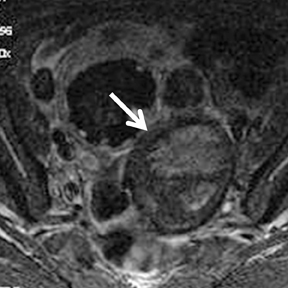
Benign cystic neoplasms and cystadenomas (serous and mucinous) make up about 30% and 20% of all ovarian tumors, respectively. Bilateral tumors are common and are more frequently with malignant.62 Serous cystadenomas are usually thin-walled, unilocular cystic lesions filled with clear fluid and have minimal septations, although these can be multilocular as well. Mucinous cystadenomas are usually larger multilocular cystic lesions filled with gelatinous material. They show different signal intensity on T1- and T2-weighted images witha “stained glass” appearance because of variable viscosity of the cyst content (Figure 10).63-65 The presence of papillary projections in both tumors raises suspicion for a borderline or malignant tumor. Contrast-enhanced studies are essential to differentiate benign from borderline or malignant tumors. These allow exact wall thickness to be measured and also demonstrate enhancing papillary projections.58,66
Benign solid tumors (Brenner, fibroma, thecoma, fibrothecoma) are all solid ovarian tumors that should be considered malignant with the
exception of a few tumors like Brenner, fibroma, thecoma, and fibrothecoma. Brenner tumor, also known as transitional cell tumor, arises from
the surface epithelium and contains dense stroma. These are mostly benign and constitute 2% to 3% of all ovarian neoplasm. Brenner tumors are usually <2 cm and are associated with other ovarian tumor in about 30% of cases when they commonly affect the ipsilateral ovary.31 Fibroma, thecoma, and fibrothecoma arise from the stromal elements of the ovary. Fibromas constitute about 4% of all ovarian neoplasm.67 If thecal elements are present, these are called fibrothecomas. These thecal cells secrete estrogen and hence tumors like fibrothecomas, thecomas may be concomitantly associated with an enlarged uterus, endometrial hyperplasia or frank endometrial carcinoma. Brenner, fibroma, and fibrothecoma are typically homogenously hypointense on T2 and intermediate to low-signal intensity on T1-weighted images. Thecoma predominantly have thecal cells and lipid laden cells without prominent fibrosis resulting in intermediate-signal intensity on T2-weighted images. Application of chemical shift imaging similar to that applied for adrenal will identify the prominent lipid component.45 When an intermediate signal is identified on T2 due to edema or degeneration contrast enhanced T1-weighted images are helpful. These tumors show no or minimal enhancement, while malignant neoplasm usually shows obvious contrast enhancement.40,52 These solid benign ovarian neoplasms may be associated with ascites and pleural effusion referred to as Meig’s syndrome.68
For a low T2 adnexal mass, the main differential to be considered is a pedunculated uterine leiomyoma. In cases with diagnostic dilemma,oblique T2-weighted images with axis perpendicular to the long axis of uterus may be helpful. This will help demonstrate the connection and relation of the mass to the uterus. Identification of a vascular pedicle showing flow voids between the uterus and mass confirms uterine leiomyoma. Additionally, leiomyoma may demonstrate a ‘claw sign’ with a rim of myometrium around the margin of the mass; however, remnants of normal ovarian tissue may also drape around an ovarian neoplasm creating a “claw sign.”
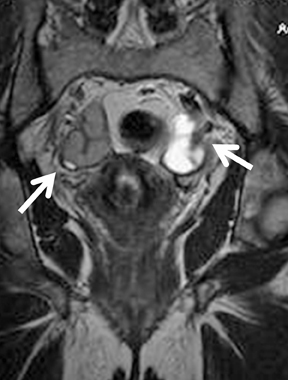
Inflammatory masses (tubo-ovarian abscess) are diagnosed clinically and with ultrasound. When acute inflammation has subsided, signs and symptoms of inflammation are less prominent and may be misdiagnosed as ovarian cancer. In such cases, MR imaging may help to make the diagnosis.69 Tubo-ovarian abscesses may demonstrate a complex solid cystic mass that enhances brightly and may have an appearance concerning for malignancy. One study has shown higher sensitivity, specificity, and accuracy of MRI of 95%, 89%, 93% respectively,in diagnosing pelvic inflammatory disease than transvaginal ultrasound (81%, 78%, 80%).70 The presence of a “halo,” an ill-defined border of the mass, stranding-of-fat plane and diffuse wall thickening are a few features that may help distinguish TOA from ovarian neoplasm.71 MR imaging helps identify the extent of inflammation from ill-defined hyperintesity on fat suppressed T2-weighted images. The abscesses may show a typical hypointense center on T1 and hyperintense center on T2 with a thick brightly enhancing wall. Purulent material however may result in variable-signal intensity on T1- and T2-weighted images (Figure 11).31,72
Malignant ovarian neoplasm is a leading cause of death among all gynecologic malignancies. CA-125 is elevated in about 80% of cases at presentation. Although ultrasound is the primary imaging modality for adnexal masses, MR imaging has shown to be the most cost effective intervention for sonogaphically indeterminate masses, according to a detailed meta-analysis.73 Contrast-enhanced MR imaging appears to be more accurate than transvaginal sonography in differentiating malignant from benign adnexal masses.74-77 MR imaging in ovarian cancer hasa high reported accuracy, ranging from 83% to 93%.78-81 The features that have been described as most suspicious for diagnosing a malignant over a benign lesion include multiple (>5) septa, irregularity, and vegetation on the wall and septum of a cystic lesion, maximal diameter >4 to6 cm, and early enhancement on DCE MR images (Figure 12).79 Hricack et al have described necrosis in a solid lesion as most predictive of malignancy in addition to vegetations in a cystic lesion.81 Spencer et al suggested assessment of a T2-weighted inhomogenous mass with contrast-enhanced T1-weighted imaging with contrast enhancement suggesting malignancy.52 Supporting features like involvement of pelvic organs or sidewall, peritoneal, mesenteric or omental disease, ascites, and adenopathy increase the sensitivity and specificity for diagnosing malignancy.82
Malignant tumors of epithelial origin represents 60% of all ovarian and 85% of all malignant ovarian neoplasm. The most common tumors in this category are serous and mucinous cystadenocarcinoma. Others include clear cell carcinoma and endometroid carcinoma.45
Serous cystadenocarcinomas are the most common variety representing about 50% of all malignant ovarian tumors. These constitute about 50% of all malignant ovarian neoplasm and are often bilateral and present as a complex solid and cystic mass. Mucinous cystadenocarcinomas constitute about 10% of all malignant ovarian tumors. These are often unilateral, larger in size with higher T1-signal intensity from high protein concentration filling the multilocuated solid cystic mass. Areas of hemorrhage and necrosis may be seen in both serous and mucinous tumors. As described before, the presence of multiple septations and papillary projections with brightly enhancing solid component help differentiate these from benign masses. In mucinous cancers, a bright component on contrast-enhanced images should be carefully compared to the T1-noncontrast images to confirm enhancement. Endometroid cancers account for approximately 15% of all malignant ovarian tumors. These tumors are mostly solid masses with areas of necrosis, usually bilateral, and often associated with endometrial hyperplasia, endometrial carcinoma or endometriosis. The diagnosis should be considered when a clearly enhancing nodule is identified in a predominantly cystic endometrioma. Clear cell cancer accounts for approximately 5% of all malignant ovarian neoplasm. These tumors generally present as a unilocular cystic mass with a solid enhancing component and can mimic serous tumors. Similar to endometroid cancer these tumors may also arise within endometrioma.
Malignant tumors of sex cord stromal origin are difficult to differentiate among the histologic subtypes based on imaging features. They constitute about 5% to 8% of all malignant ovarian neoplasm and include granulosa cell tumor (arise from primitive sex cords) and Sertoli-Leydig cell tumor (arise from theca and Leydig cells). Most tumors exhibit estrogenic effects. Granulosa cell tumors can be seen both in prepubertal (juvenile subtype) and peri or postmenopausal (adult subtype) females. They can present with abnormal vaginal bleeding and can be associated with endometrial hyperplasia, polyps, and carcinoma.These tumors are confined to the ovary, are rarely bilateral, and are associated with an excellent prognosis. They can range from entirely solid to completely cystic tumors.83 These tumors have rich fibrous stroma resulting in a low-signal intensity of T2WI. They typically appear as a sponge-like multilocular cystic mass filled with blood clots.84,85
Malignant tumors of germ cell origin account for approximately 5% of all malignant ovarian neoplasm and occur in the first 2 decades of life.These include dysgerminoma, endodermal sinus tumor, and immature teratoma. Dysgerminomas have an excellent prognosis. They present characteristically as multilobulated solid masses with prominent fibrovascular septa. Speckled calcification may be present.86 Endodermal sinus tumor, also known as yolk sac tumor, is associated with a poor prognosis. They present as large, complex pelvic masses that extend into the abdomen containing both solid and cystic components. Most patients have an elevated alpha-fetoprotein level.87,88 Immature teratoma is a rare germ cell tumor containing tissue from all 3 germ cell layers. The tumor capsule is generally perforated and hence not always identified. These tumors demonstrate both cystic and solid components with scattered calcifications and small foci of fat.89,90
Ovarian metastasis can arise from intestinal tumors, the stomach most commonly, breast cancer via hematogenous or lymphatic spread orserosal implantation of cells shed into peritoneal cavity. The term ‘Krukenberg tumor’ specifically refers to tumors with malignant mucin-filled signet ring cells within the ovarian stroma. Krukenberg tumors are generally bilateral and have both solid and cystic component with varying T1 and T2 signal.31 Ectopic pregnancy (EP) is usually diagnosed by transvaginal sonography. MR imaging seems to have an advantage in its ability to accurately localize and provide better delineation of the focus owing to its excellent tissue contrast for indeterminate cases. MR imaging findings include hematosalpinx, enhancement of a fallopian tube wall, a gestation sac-like cystic structure, bloody ascites and frequently surrounding acute hematoma of distinct low intensity on T2-weighted images.91 A study by Yoshigi et al concluded that MRI using T(2)*-WI is a sensitive, specific,and accurate method to evaluate EP because of its sensitivity to fresh hematoma.92
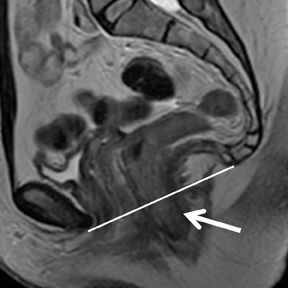
Pelvic floor imaging is utilized to accurately detect if specific pelvic dysfunction like stress urinary incontinence (SUI), pelvic organ prolapse (POP), pelvic pain or pressure, and anal incontinence are associated with specific pelvic floor abnormalities. MR is the imaging modality of choice for the evaluating pelvic floor because of its superior visualization of pelvic musculature, fascial planes, and the visceral organs that constitute the pelvic floor.93,94 It has been suggested that identifying a predominant anatomic defect on static images and visualizing corresponding implication on the kinematics of the pelvic organ on dynamic sequences would allow a defect-specific surgical approach to pelvic floor dysfunction for each patient. Dynamic imaging can be performed in different phases while the patient is at rest, during pelvic floor contraction,during variable levels of stress, and with a Valsalva maneuver (Figure 13).95 Hecht et al suggested inclusion of near real-time continuous imaging with a dynamic true fast imaging with steady-state precession (FISP) sequence in addition to dynamic multiplanar HASTE sequences to evaluate pelvic floor dysfunction.96
Acute pelvic pain during pregnancy is usually assessed by sonography initially. In pregnant patients sonographic evaluation can sometimes be limited by the gravid uterus and gas in the bowel. MR imaging, with its superior contrast resolution and without fetal exposure to ionizing radiation, can be used as an effective problem solving alternative. The safety of MR imaging during pregnancy is not yet established, although a few studies in humans have shown no long-term adverse effect in children who underwent MR imaging as fetuses.97-100 Various indications for pelvic MR may include, but are not limited to, gynecologic conditions such as hemorrhagic ovarian cysts, pelvic inflammatory disease, ovarian torsion, ectopic pregnancy, an impending miscarriage secondary to fetal distress or demise, and placental abruption, and to nongynecologic etiologies, such as appendicitis, inflammatory bowel disease, infectious enteritis, diverticulitis, urethral calculi, pyelonephritis, and pelvic thrombophlebitis.
According to American College of Radiology (ACR) appropriateness criteria, when a gynecologic condition is suspected, MR of pelvis in acute pelvic pain in pregnant patient may be appropriate when ultrasound is inconclusive or nondiagnostic. When a nongynecologic condition is suspected MRI is usually appropriate and it was rated as ‘8’ with ‘9’ being considered the most appropriate study. Nevertheless, the recommendation for administering gadolinium contrast agents to perform an in-depth analysis of benefit to mother and fetus versus risk of fetal exposure to free gadolinium ions that may stay in the amniotic fluid after excretion from fetal kidneys for an indeterminate time.101
Conclusion
Thorough knowledge of the spectrum of MR imaging features of various physiologic variations and pathologic conditions that affect the female pelvis is essential for establishing an accurate diagnosis and guiding further management. MR is a reliable staging method for preoperative assessment of endometrial and cervical carcinoma. MR imaging allows accurate distinction between various benign and malignant uterine and ovarian conditions when ultrasound is indeterminate. It can serve as an adjunct to diagnostic laproscopy, hysteroscopy, hysterosalpingography, and transvaginal USG in patients being evaluated for infertility.
References
- Wang J, Yu T, Bai R, et al. The value of the apparent diffusion coefficient in differentiating stage IA endometrial carcinoma from normal endometrium and benign diseases of the endometrium: Initial study at 3-T magnetic resonance scanner. J Comput Assist Tomogr. 2010;34:332-337.
- Inada Y, Matsuki M, Nakai G, et al. Body diffusion-weighted MR imaging of uterine endometrial cancer: Is it helpful in the detection of cancer in nonenhanced MR imaging? Eur J Radiol. 2009;70:122-127.
- Takeuchi M, Matsuzaki K, Nishitani H. Manifestations of the female reproductive organs on MR images: Changes induced by various physiologic states. Radiographics. 2010;30:1147.
- Huertas CP, Brown MA, Semelka RC. MR imaging evaluation of the adnexa. Magn Reson Imaging Clin N Am. 2006;14:471-487, vi.
- Morisawa N, Koyama T, Kido A, et al. Physiologic changes of the normal ovaries on T2-weighted images and diffusion-weighted images during the menstrual cycle. Proc Intl Soc Mag Reson Med. 2007;15:572.
- Outwater EK, Talerman A, Dunton C. Normal adnexa uteri specimens: Anatomic basis of MR imaging features. Radiology. 1996;201:751-755.
- Outwater EK, Mitchell DG. Normal ovaries and functional cysts: MR appearance. Radiology. 1996;198:397-402.
- Heilbrun ME, Olpin J, Shaaban A. Imaging of benign adnexal masses: Characteristic presentations on ultrasound, computed tomography, and magnetic resonance imaging. Clin Obstet Gynecol. 2009;52:21-39.
- Chapron C, Dubuisson JB, Fritel X, Rambaud D. Diagnosis and management of organic ovarian cysts: Indications and procedures for laparoscopy. Hum Reprod Update. 1996;2:435446.
- Audebert AJ. Laparoscopic surgery for ovarian cysts. Curr Opin Obstet Gynecol. 1996;8:261-265.
- Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-46.
- Rajkotia K, Veeramani M, Macura KJ. Magnetic resonance imaging of adnexal masses. Top Magn Reson Imaging. 2006;17:379-397.
- Lee SI. Radiological reasoning: Imaging characterization of bilateral adnexal masses. AJR Am J Roentgenol. 2006;187:S460-466.
- Outwater EK, Dunton CJ. Imaging of the ovary and adnexa: Clinical issues and applications of MR imaging. Radiology. 1995;194:1-18.
- Vessey M, Metcalfe A, Wells C, et al. Ovarian neoplasms, functional ovarian cysts, and oral contraceptives. Br Med J. (Clin Res Ed). 1987;294:1518-1520.
- Stany MP, Hamilton CA. Benign disorders of the ovary. Obstet Gynecol Clin North Am. 2008;35:271-284, ix. Review.
- Tamai K, Koyama T, Saga T, et al. MR features of physiologic and benign conditions of the ovary. Eur Radiol. 2006;16:2700-2711. Review.
- Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. Radiographics. 2000;20:1445-1470.
- Togashi K. MR imaging of the ovaries: Normal appearance and benign disease. Radiol Clin North Am. 2003;41:799-811.
- Valentin L, Skoog L, Epstein E. Frequency and type of adnexal lesions in autopsy material from postmenopausal women: ultrasound study with histological correlation. Ultrasound Obstet Gynecol. 2003;22:284-289.
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women >55 years old in a large cancer screening trial. Am J Obstet Gynecol. 2010;202:373.e1-9.
- Castillo G, Alcázar JL, Jurado M. Natural history of sonographically detected simple unilocular adnexal cysts in asymptomatic postmenopausal women. Gynecol Oncol. 2004;92:965-969.
- McDonald JM, Modesitt SC. The incidental postmenopausal adnexal mass. Clin Obstet Gynecol. 2006;49:506-516.
- Modesitt SC, Pavlik EJ, Ueland FR, et al. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102:594-599.
- Spencer JA, Ghattamaneni S. MR imaging of the sonographically indeterminate adnexal mass. Radiology. 2010;256:677-694.
- Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: diagnosis with MR imaging. Radiology. 1991;180:73-78.
- Outwater E, Schiebler ML, Owen RS, Schnall MD. Characterization of hemorrhagic adnexal lesions with MR imaging: Blinded reader study. Radiology. 1993;186:489–494.
- Sugimura K, Okizuka H, Imaoka I, et al. Pelvic endometriosis: Detection and diagnosis with chemical shift MR imaging. Radiology. 1993;188:435-438.
- Jain KA, Jeffrey RB. Evaluation of pelvic masses with magnetic resonance imaging and ultrasonography. J Ultrasound Med. 1994;13:845-853.
- Jain KA, Friedman DL, Pettinger TW, et al. Adnexal masses: Comparison of specificity of endovaginal US and pelvic MR imaging. Radiology. 1993;186:697-704.
- Imaoka I, Wada A, Kaji Y, et al. Developing an MR imaging strategy for diagnosis of ovarian masses. Radiographics. 2006;26:1431-1448.
- Imaoka I, Sugimura K, Okizuka H, et al. Ovarian cystic teratomas: Value of chemical fat saturation magnetic resonance imaging. Br J Radiol. 1993;66:994-997.
- Stevens SK, Hricak H, Campos Z. Teratomas versus cystic hemorrhagic adnexal lesions: Differentiation with proton-selective fat-saturation MR imaging. Radiology. 1993;186:481-488.
- Kido A, Togashi K, Konishi I, et al. Dermoid cysts of the ovary with malignant transformation: MR appearance. AJR Am J Roentgenol. 1999;172:445-449.
- Brant WE, Helms CA. Fundamentals of diagnostic radiology, 3rd Ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2007.
- Jung SE, Lee JM, Rha SE, et al. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics. 2002;22: 1305-1325.
- Ghossain MA, Buy JN, Lignères C, et al. Epithelial tumors of the ovary: Comparison of MR and CT findings. Radiology. 1991;181:863-870.
- Tanaka YO, Nishida M, Kurosaki Y, et al. Differential diagnosis of gynaecological “stained glass” tumours on MRI. Br J Radiol. 1999;72:414-420.
- Bazot M, Nassar-Slaba J, Thomassin-Naggara I, et al. MR imaging compared with intraoperative frozen-section examination for the diagnosis of adnexal tumors; Correlation with final histology. Eur Radiol. 2006;16:2687-2699.
- Sutton CL, McKinney CD, Jones JE, Gay SB. Ovarian masses revisited: Radiologic and pathologic correlation. Radiographics. 1992;12:853-877.
- Chang WC, Meux MD, Yeh BM, et al. CT and MRI of adnexal masses in patient with primary nonovarian malignancy. AJR Am J Roentgenol. 2006;186:1039-1045.
- Ha HK, Lim GY, Cha ES, et al. MR imaging of tubo-ovarian abscess. Acta Radiol. 1995;36:510-514.
- Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: Comparison with laparoscopy and US. Radiology. 1999;210:209-216.
- Togashi K. Ovarian cancer: the clinical role of US, CT, and MRI. Eur Radiol. 2003;13:L87-104.
- Kim MY, Rha SE, Oh SN, et al. MR Imaging findings of hydrosalpinx: A comprehensive review. Radiographics. 2009;29:495-507.
- Kinkel K, Lu Y, Mehdizade A, et al. Indeterminate ovarian mass at US: Incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology. 2005;236:85-94.
- Yamashita Y, Torashima M, Hatanaka Y, et al. Adnexal masses: Accuracy of characterization with transvaginal US and precontrast and postcontrast MR imaging. Radiology. 1995;194:557-565.
- Komatsu T, Konishi I, Mandai M, et al. Adnexal masses: Transvaginal US and gadolinium-enhanced MR imaging assessment of intratumoral structure. Radiology. 1996;198:109-115.
- Hata K, Hata T, Manabe A, et al. A critical evaluation of transvaginal Doppler studies, transvaginal sonography, magnetic resonance imaging, and CA 125 in detecting ovarian cancer. Obstet Gynecol. 1992;80:922-926.
- Yamashita Y, Hatanaka Y, Torashima M, et al. Characterization of sonographically indeterminate ovarian tumors with MR imaging. A logistic regression analysis. Acta Radiol. 1997;38:572-577.
- Bazot M, Nassar-Slaba J, Thomassin-Naggara I, et al. MR imaging compared with intraoperative frozen-section examination for the diagnosis of adnexal tumors; correlation with final histology. Eur Radiol. 2006;16:2687-2699.
- Sohaib SA, Sahdev A, Van Trappen P, et al. Characterization of adnexal mass lesions on MR imaging. AJR Am J Roentgenol. 2003;180: 1297-1304.
- Rieber A, Nüssle K, Stöhr I, et al. Preoperative diagnosis of ovarian tumors with MR imaging: Comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Am J Roentgenol. 2001;177:123-129.
- Hricak H, Chen M, Coakley FV, et al. Complex adnexal masses: Detection and characterization with MR imaging--multivariate analysis. Radiology. 2000;214:39-46.
- Stevens SK, Hricak H, Stern JL. Ovarian lesions: Detection and characterization with gadolinium-enhanced MR imaging at 1.5 T. Radiology. 1991;181:481-488.
- Jung SE, Rha SE, Lee JM, et al. CT and MRI findings of sex cord-stromal tumor of the ovary. AJR Am J Roentgenol. 2005;185:207-215. 83.Tanaka YO, Saida TS, Minami R, et al. MR findings of ovarian tumors with hormonal activity, with emphasis on tumors other than sex cord-stromal tumors. Eur J Radiol. 2007;62:317-327.
- Kim SH, Kim SH. Granulosa cell tumor of the ovary: Common findings and unusual appearances on CT and MR. J Comput Assist Tomogr. 2002;26:756-761. Erratum in: J Comput Assist Tomogr. 2003;27:103.
- Kim SH, Kang SB. Ovarian dysgerminoma: Color Doppler ultrasonographic findings and comparison with CT and MR imaging findings. J Ultrasound Med. 1995;14:843-848.
- Levitin A, Haller KD, Cohen HL, et al. Endodermal sinus tumor of the ovary: Imaging evaluation. AJR Am J Roentgenol. 1996;167:791-793.
- Yamaoka T, Togashi K, Koyama T, et al. Yolk sac tumor of the ovary: Radiologic-pathologic correlation in four cases. J Comput Assist Tomogr. 2000;24:605-609.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: Tumor types and imaging characteristics. Radiographics. 2001;21:475-490.
- Brammer HM 3rd, Buck JL, Hayes WS, et al. From the archives of the AFIP. Malignant germ cell tumors of the ovary: radiologic-pathologic correlation. Radiographics. 1990;10:715-724.
- Tamai K, Koyama T, Togashi K. MR features of ectopic pregnancy. Eur Radiol. 2007;17:3236-3246.
- Yoshigi J, Yashiro N, Kinoshita T, et al. Diagnosis of ectopic pregnancy with MRI: Efficacy of T2*-weighted imaging. Magn Reson Med Sci. 2006;5:25-32.
- Boyadzhyan L, Raman SS, Raz S. Role of static and dynamic MR imaging in surgical pelvic floor dysfunction. Radiographics. 2008;28:949-967.
- Martin DR, Salman K, Wilmot CC, Galloway NT. MR imaging evaluation of the pelvic floor for the assessment of vaginal prolapse and urinary incontinence. Magn Reson Imaging Clin N Am. 2006;14:523-535.
- El Sayed RF, El Mashed S, Farag A, et al. Pelvic floor dysfunction: assessment with combined analysis of static and dynamic MR imaging findings. Radiology. 2008;248:518-530.
- Hecht EM, Lee VS, Tanpitukpongse TP, et al. MRI of pelvic floor dysfunction: Dynamic true fast imaging with steady-state precession versus HASTE. AJR Am J Roentgenol. 2008;191:352-358.
- Baker PN, Johnson IR, Harvey PR, et al. A three-year follow-up of children imaged in utero with echo-planar magnetic resonance. Am J Obstet Gynecol. 1994;170:32-33.
- Glover P, Hykin J, Gowland P, et al. An assessment of the intrauterine sound intensity level during obstetric echo-planar magnetic resonance imaging. Br J Radiol. 1995;68:1090-1094.
- Clements H, Duncan KR, Fielding K, et al. Infants exposed to MRI in utero have a normal paediatric assessment at 9 months of age. Br J Radiol. 2000;73:190-194.
- Kok RD, de Vries MM, Heerschap A, van den Berg PP. Absence of harmful effects of magnetic resonance exposure at 1.5 T in utero during the third trimester of pregnancy: A follow-up study. Magn Reson Imaging. 2004;22:851-854.
- ACR Appropriateness Criteria® acute pelvic pain in the reproductive age group, released 2008; http://www.acr.org/acet/Acute-Abdomina-Pain-Fever-ET.pdf.
Related Articles
Citation
. Part 2: MR of the female pelvis. Appl Radiol.
April 27, 2012