Pediatric bone imaging: Differentiating benign lesions from malignant
Images






This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Bone lesions are commonly encountered in pediatric patients, with primary bone tumors representing the 6th most common neoplasm.1 Fortunately, most pediatric bone tumors are benign.2 Although cross-sectional imaging such as CT or MRI can be useful, plain film continues to be the primary modality in the initial evaluation of osseous abnormalities. The most common pediatric benign bone tumors include nonossifying fibroma (NOF), osteochondroma, Langerhans cell histiocytosis (LCH), unicameral bone cyst (UBC), aneurysmal bone cyst (ABC), and osteoid osteoma.3,4
The most common malignant pediatric bone tumors include osteosarcoma and Ewing sarcoma.1 Differentiating between benign versus malignant bone tumors is not always straightforward. There are, however, radiographic characteristics that can help differentiate between the two, including tumor margin, periosteal reaction, and bone destruction. The presence of a soft-tissue mass is uncommon in benign tumors. Peritumoral edema can be misleading, as it can be seen in both benign and malignant lesions. In addition, matrix type and tumor location can help narrow the differential diagnosis.
The main teaching point of this article is to help the reader understand, recognize, describe and differentiate the characteristics and features of common benign and malignant bone lesions.
Lesion characteristics
Margins
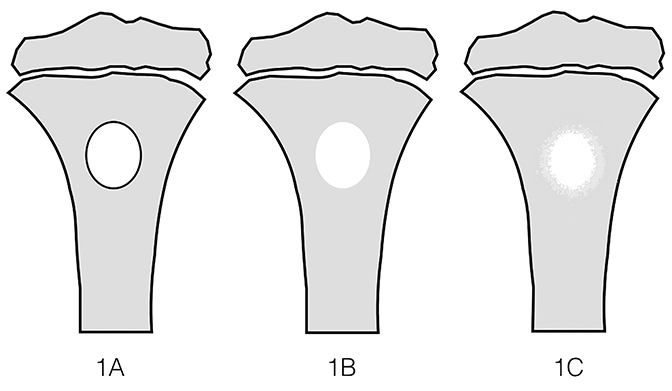
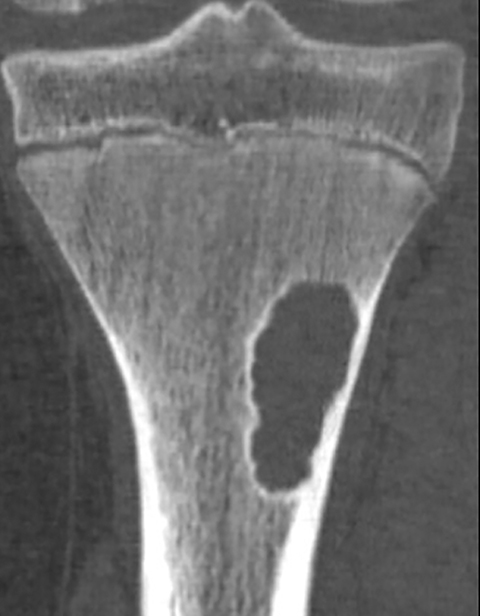
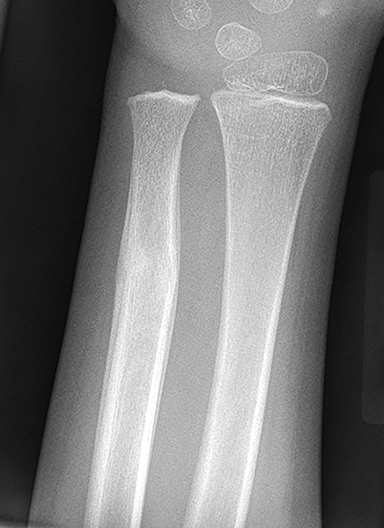
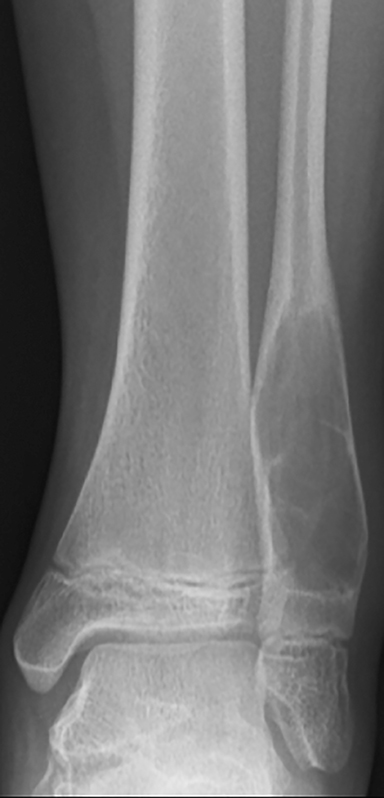
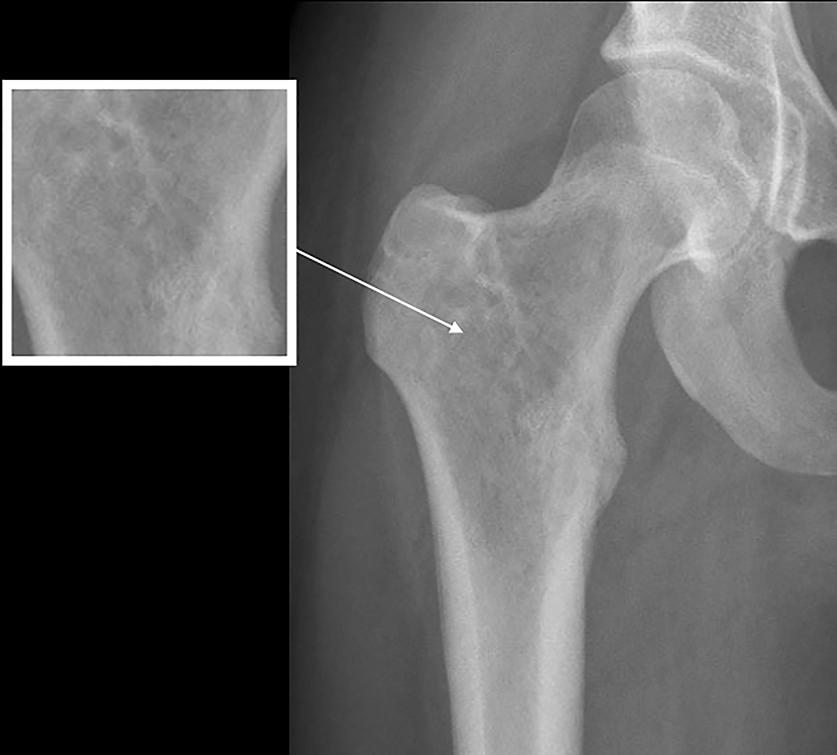
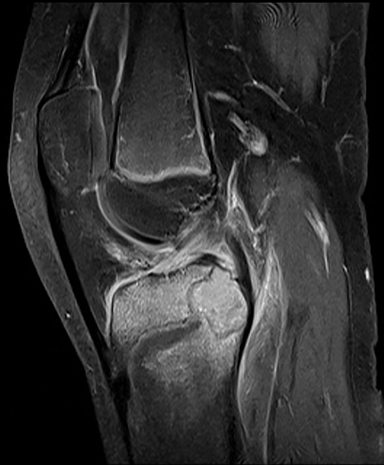
Tumor margins are an important factor in differentiating between a benign versus malignant process (Figure 1). Sharply demarcated margins (1A and 1B) are described as those that you can easily draw around with a pencil. A sharp border indicates a tumor is slow growing, which suggests benignity.3 1A margins are sharply defined with a sclerotic border, which is typical of NOF (Figure 2) and UBC. 1B margins are sharply defined without a sclerotic border. 1B margins are also referred to as sharply lytic, which can be seen in ABCs (Figure 3) and LCH.5 Conversely, 1C margins are poorly defined or indistinct, described as having a wide zone of transition. This indicates a fast-growing lesion, which is more suggestive of a malignant or aggressive process. 1C margins are characteristic of aggressive tumors such as Ewing sarcoma (Figure 4) or osteosarcoma, but can also be seen in osteomyelitis.6
Periosteal reaction
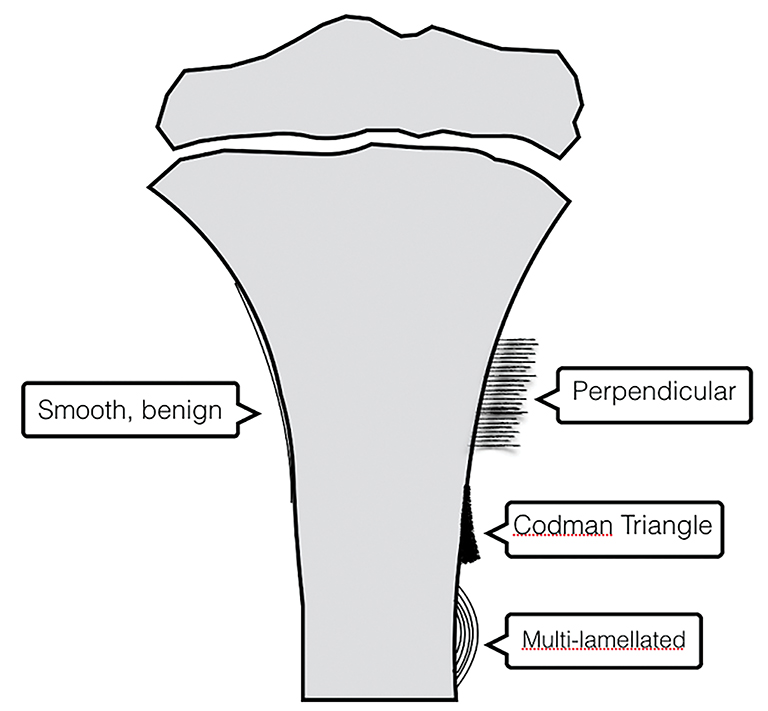
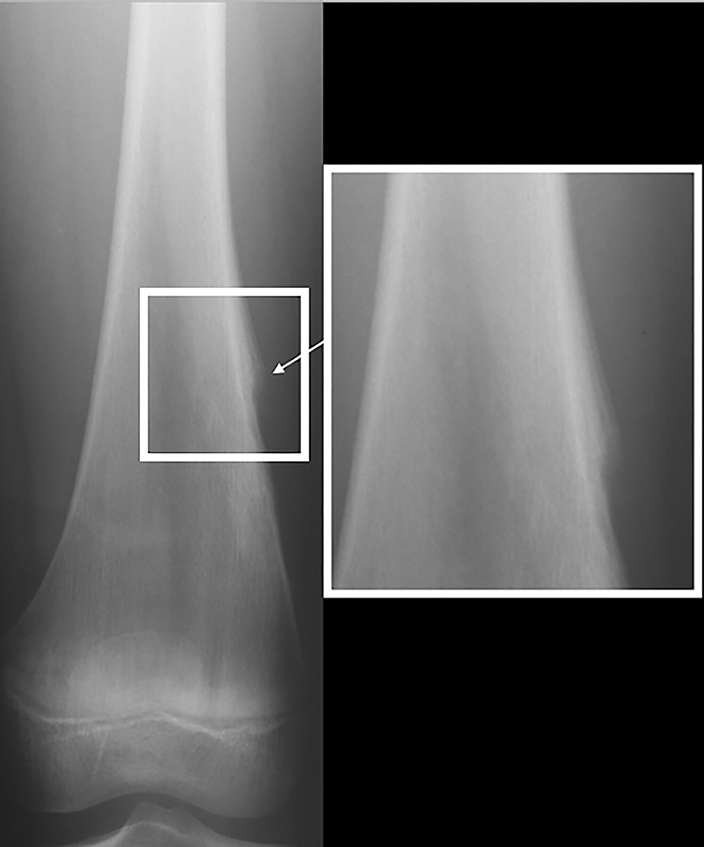
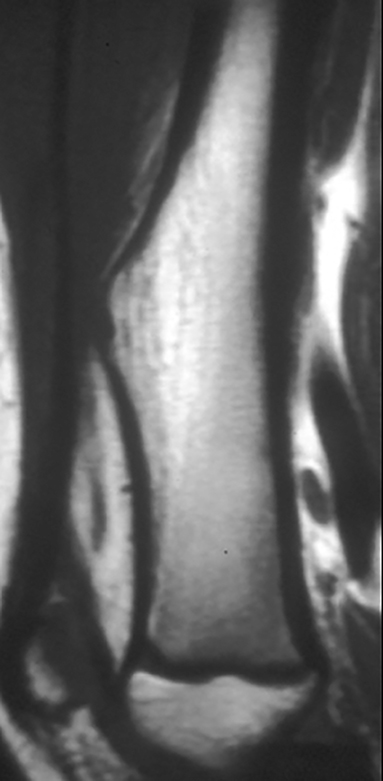
Periosteal reaction results from cortical insult.7 It is not specific to bone tumors; however, it can help distinguish benignity versus malignancy (Figure 5). Smooth, uninterrupted periosteal reaction is seen in non-aggressive, benign tumors (Figure 6). In contrast, irregular or interrupted periosteal reaction is seen in aggressive or malignant tumors. “Sunburst” or perpendicular periosteal reaction (Figure 7) and “onion-skin” or multi-lamellated (Figure 8) periosteal reaction are commonly described in malignant tumors. Codman triangle (Figure 9), where the periosteum is lifted off the cortex by the tumor with central interruption, is also associated with malignancy.7
Bone destruction
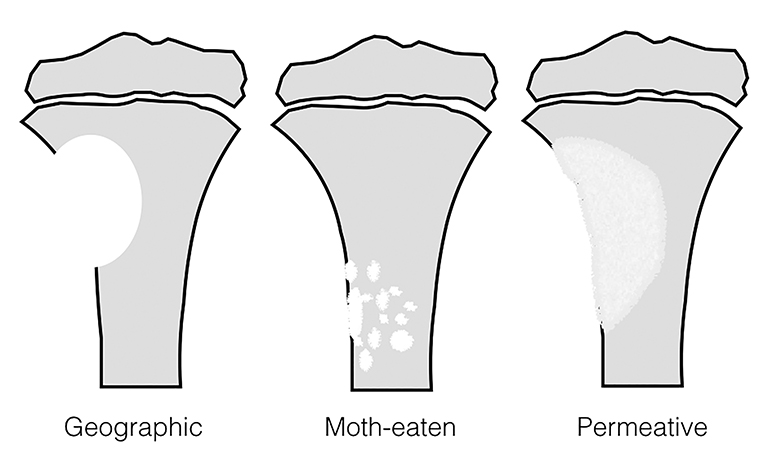
The pattern of bone destruction is related to the growth of a tumor (Figure 10). Benign tumors, which are typically slow growing, display geographic bone destruction. Geographic bone destruction can be seen in UBCs (Figure 11), LCHs, enchondromas, and GCTs.3 Malignant tumors demonstrate rapid growth and infiltration causing more aggressive bone destruction. Moth-eaten (Figure 4) and permeative (Figures 7, 12) bone destruction are described in malignant bone tumors such as Ewing sarcoma and osteosarcoma, but can be seen with osteomyelitis.
Soft-tissue mass
The presence of a soft-tissue mass almost always suggests a malignant process.5 The rare exceptions in which a benign tumor may have soft tissue involvement include ABC and GCT. A soft tissue component is frequently seen with both osteosarcoma and Ewing sarcoma, but it is much more pronounced than the osseous involvement in Ewing sarcoma, which may aid in differentiating between them.6
Peritumoral edema
Peritumoral edema, best evaluated on MRI with fat-suppressed fluid-sensitive sequences, can be very misleading when trying to decide if a tumor is benign or malignant. Although osteosarcoma and Ewing sarcoma demonstrate bone marrow edema, so do benign tumors such as osteoid osteoma, chondroblastoma, and LCH.4 Another drawback of peritumoral edema is that the increased T2 signal can obscure the true margins of the lesion, necessitating the use of T1 sequences. It is for this reason that plain film should always be included in a bone tumor work-up rather than relying on MRI alone.8
Matrix
The internal composition of a bone tumor can help narrow the differential diagnosis. Matrix type depends on the material produced by the mesenchymal cells of the tumor. The most common types of matrix are chondroid and osteoid.3 Chondroid matrix calcifications are described as “ring-and-arcs,” which can be seen with enchondromas (Figure 13) and chondroblastomas. Osteoid matrix is described as “cloud-like,” which can be seen in osteosarcomas (Figure 7).
Location
Bone tumors often have a propensity for certain locations within the bone, which can help with the differential diagnosis (Figure 14). Epiphyseal tumors are uncommon overall, and only a couple tumors tend to occur in this location. If the physes are open, the most likely diagnosis is chondroblastoma (Figure 15). If the physes have closed, the leading differential diagnosis is GCT (Figure 16), which nearly always extends to the articular margin of a bone.3 Differentiating between metaphyseal and diaphyseal origin of a lesion is not always easy. Some tumors begin in the metaphysis, but end up in the diaphysis from skeletal growth. UBCs, ABCs and enchondromas can occur in either the metaphysis or diaphysis. Whereas NOF tends to occur only in the metaphysis, and osteosarcoma favors the metadiaphysis. Ewing sarcoma typically occurs in the diaphysis.
The location of the tumor in relation to the central axis of the bone can also help narrow the differential diagnosis. For example, UBC, LCH, and enchondromas are typically located centrally in the bone. ABCs are typically eccentric, but can be central when in a small long bone such as the fibula. Eccentric lesions include NOF, GCT, and osteosarcoma. Osteoid osteoma is a cortically based lesion.
Benign tumor
Unicameral bone cyst
UBCs (Figure 11), also known as simple bone cysts, are tumor-like lesions of unknown origin, thought to be developmental rather than true neoplasms.3 UBCs represent up to 8% of benign bone tumors, and they are most common in the first two decades.2,9 They are often asymptomatic and discovered incidentally, unless there is an associated pathological fracture. A “fallen fragment” sign, which is described as a piece of cortex falling into the cystic cavity, is pathognomonic for a UBC.3, 4 Radiographically, a UBC is a well-defined radiolucent lesion with 1A or 1B margins, centrally located within the metaphysis and/or diaphysis. They are commonly located within the proximal humerus and femur. On MRI, UBCs have fluid signal intensity, and typically demonstrate a thin rim of enhancement on post contrast images.4 UBCs are typically uniloculated, but may show fluid-fluid levels, which suggests prior hemorrhage.4,8
Aneurysmal bone cyst
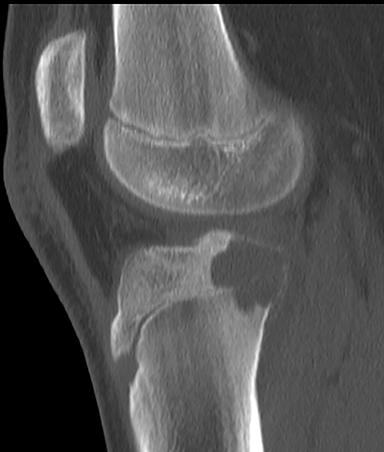
Aneurysmal bone cysts (ABCs, Figure 3) represent approximately 8% of benign bone tumors and are most common the first two decades.3,9 They may arise de novo in bone, or may occur secondary to trauma or in association with another lesion such as a chondroblastoma or fibrous dysplasia.3,4 Radiographically, an ABC is an eccentric, multi-cystic, expansile lesion with well-defined 1A or 1B margins. They may exhibit benign periosteal reaction and are commonly located in the metaphysis. If the periosteum is bulging, a soft tissue “mass” can be produced.3 An ABC can be a difficult benign tumor to assess because of the similarity to the rare telangiectatic osteosarcoma.8 The best plain film or CT finding to help distinguish benignity is a narrow zone of transition with well-defined borders. On MRI, an ABC demonstrates fluid signal intensity with multiple fluid-fluid levels, multiple septations that typically enhance and an intact rim, unless traumatized.3
Osteoid osteoma
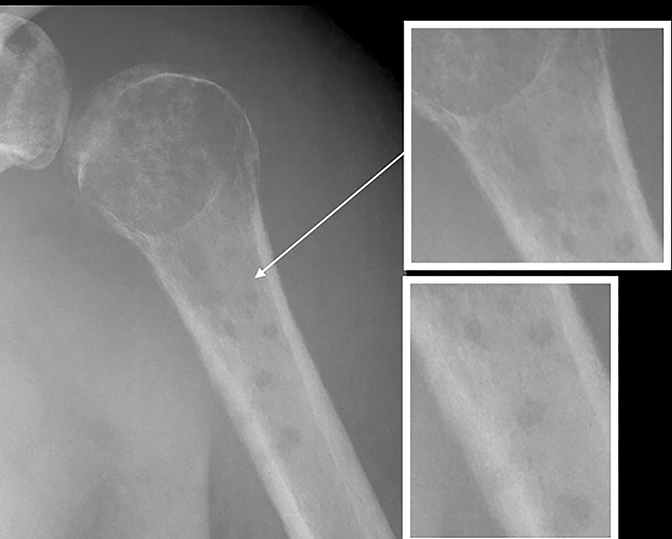
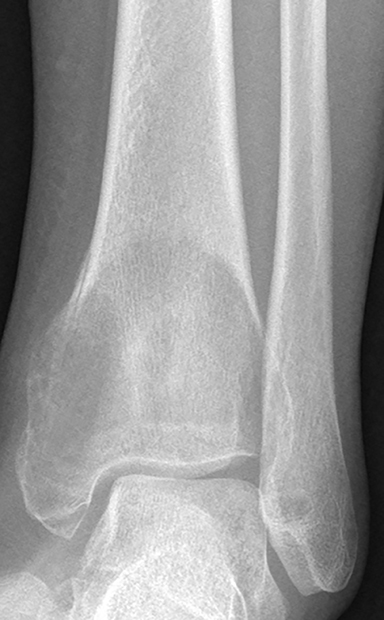
Osteoid osteomas (Figure 17) represent 8% of benign bone tumors and are most common in the second decade.3,9 The most common clinical presentation is pain at night relieved by NSAIDs. Classically, they are described as cortically based, diaphyseal or metaphyseal with a radiolucent nidus within an area of surrounding sclerosis.3,8 Benign periosteal reaction (Figure 6) or cortical thickening is frequently seen. Osteoid osteoma can occur in any bone, but mostly occur in long bones. The radiolucent nidus may demonstrate a focal calcification.3 CT is the modality of choice to accurately identify the nidus for definitive diagnosis.3,8 MRI is not preferred because surrounding bone marrow edema may obscure the nidus, leading to the wrong diagnosis.
Enchondroma
Enchondroma (Figure 14) is the second-most common benign bone tumor, accounting for 10% of all such lesions.3,9 They are most common between the 3rd and 5th decade.3 These cartilaginous tumors contain chondroid matrix with “ring-and-arc” or “popcorn” calcifications and are centrally located with endosteal scalloping.3,4 They frequently occur in the short tubular bones of the hands and feet, and they may assume more of a well-defined lytic and expansile appearance.3 On MRI, enchondromas tend to be lobular in contour and demonstrate high T2 signal. They may have small foci of low signal, which is related to the calcification in the chondroid matrix.3,4 Although there is a possibility of malignant transformation, this is very uncommon in the pediatric population.4
Osteochondroma
Osteochondromas also referred to as exostosis, are the most common benign bone tumors and account for 21-45% of such lesions (Figure 18).3,9 They are most common in the first three decades.3 Osteochondromas are of cartilaginous origin and are commonly located in the metaphysis of long bones.8 They are described as either pedunculated (having a long stalk) or sessile (having a flat base). These lesions arise from the surface of the bone, demonstrating continuity with the medullary cavity (Figure 18), and characteristically have a cartilage cap. Pedunculated osteochondromas point away from the joint, which can help distinguish them from a supracondylar process of the elbow. On MRI, the cartilage cap thickness can also be evaluated and normal bone marrow will be seen extending into the osteochondroma. Surrounding soft tissue edema or focal bursal formation can be seen if there is trauma or local friction.
Nonossifying fibroma
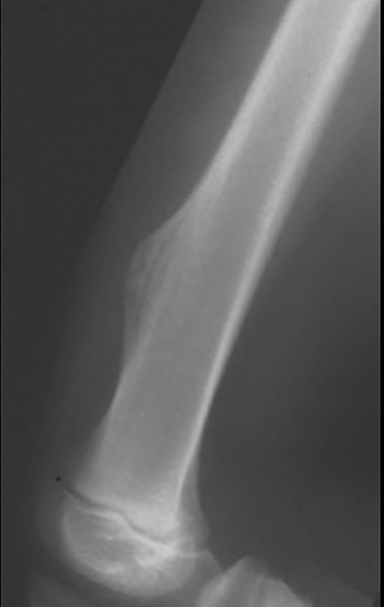
Nonossifying fibroma (NOF, Figure 2), also referred to as fibrous cortical defect if less than 2 cm in size, is the most common benign lesion in children.4,8 They can be seen in up to 30% of the general population and are most common in the 1st decade.3 They are eccentrically located, usually within the metaphysis of long bones, particularly around the knee.8 Nonossifying fibromas demonstrate a radiolucent center with a sclerotic well-defined 1A margin. They are usually asymptomatic and found incidentally, unless there is an associated pathological fracture. On MRI, NOF demonstrates T2 hyperintensity during the development phase, and progresses to low signal intensity on T1 and T2 sequences as it matures. Contrast enhancement is not uncommon and tends to be greater in immature or developing lesions than longstanding NOFs.8
Langerhans cell histiocytosis
Langerhans cell histiocytosis (LCH, Figure 19), also known as eosinophic granuloma, represents less than 1% of biopsy-proven primary bone lesions.3 They can be seen in the first few months of life through the 8th decade, but the mean age is 5-10 years.3 These are lytic lesions that may have either a wide or narrow zone of transition, and may even demonstrate a more permeative pattern of bone destruction.4 There is a propensity for location in the calvarium, pelvis, ribs, and long bones such as the femur. Within long bones, LCH is typically metaphyseal or diaphyseal and may be associated with periosteal reaction. MRI appearance is variable, but the most common presentation is a focal lesion with surrounding bone marrow edema.3
Chondroblastoma
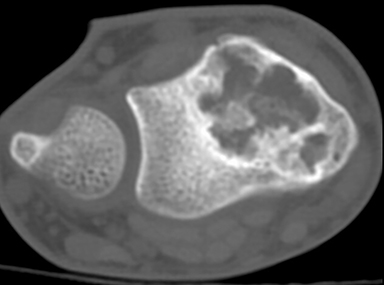
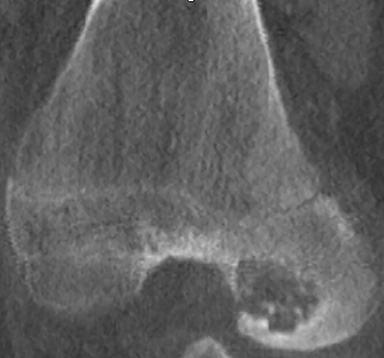
Chondroblastoma (Figures 15, 20) is a benign tumor of immature cartilage, which accounts for 3% of all benign bone tumors.9 They are most common in the 2nd decade.3 It is characteristically located in the epiphysis or in an apophysis, but may extend into the metaphysis if it develops after physeal closure.8 Radiographically, it is an eccentrically located, radiolucent lesion with a thin well-defined sclerotic 1A margin and geographic pattern of bone destruction. MRI can be misleading because of local reaction including peritumoral marrow edema, soft tissue edema and inflammation (Figure 20), and joint effusion, which suggests a more aggressive lesion, unlike the plain film exam. Chondroblastomas have a peripheral thin hypointense rim, which corresponds to the sclerotic margin seen on plain film or CT.4
Giant cell tumor
GCTs (Figure 17) make up 14-22 % of all benign bone tumors and are most common in the 3rd to 5th decades.3,9 They occur almost exclusively after physeal closure. Radiographically, GCTs are eccentrically located radiolucent lesions with well-defined lytic 1B margins and geographic bone destruction. In skeletally mature patients, GCTs begin in the metaphysics and extend deep to the subchondral bone plate of the articular surface. On MRI, GCTs exhibit inhomogeneous increased T2 signal and inhomogeneous enhancement on postcontrast images.
Malignant
Osteosarcoma
Osteosarcoma (Figure 7) is the most common primary malignant bone tumor, representing 20% of all primary malignant bone tumors.3,4 There is a bimodal distribution with most occurring in the 1st and 2nd decade and a second peak after the 7th decade.3 The most common subtype is an intramedullary osteoid producing tumor, which occurs at the metadiaphysis of long bones, frequently around the knee.4,6 Radiographically, osteosarcomas are destructive, eccentrically located lesions with poorly defined 1C margins and an associated soft-tissue mass. They frequently have osteoid or “cloud-like” bone formation and aggressive periosteal reaction, classically a sun-burst or Codman triangle pattern. Osteosarcomas may have skip lesions, which are osseous or marrow metastasis within the same bone or adjacent bones in relation to the dominant lesion.6 MRI is helpful in determining extent of both the bone tumor and the associated soft-tissue mass.6 MRI can also help identify skip lesions and evaluate for the presence or absence of neurovascular bundle involvement.4
Ewing sarcoma
Ewing sarcoma (Figure 12) is the second-most common primary malignant bone tumor in the pediatric population, representing 8% of all primary malignant bone tumors.4,9 Most occur in the 1st and 2nd decade.3 They can have a variable radiographic appearance. Ewing sarcoma typically occurs in the diaphysis of the long bones with moth-eaten or permeative bone destruction and an aggressive periosteal reaction, classically known as the “onion-skin” pattern. It does not produce an osteoid matrix, which is a key differentiation from osteosarcoma.4 MRI is important in evaluating Ewing sarcoma because it classically has a large soft tissue component, more pronounced than the osseous destruction.6 Post-gadolinium images demonstrate more pronounced tumoral enhancement, which aids in distinguishing tumor from the surrounding marrow edema.3
Conclusion
Bone tumors are one of the most common lesions encountered by radiologists. Distinguishing between benign and malignant tumors is possible by carefully evaluating the lesion’s characteristics such as type of margin, pattern of bone destruction, type of periosteal reaction and presence of an associated soft-tissue mass. Using the location of the lesion, type of matrix, and patient age can help narrow the differential diagnosis. The most important modality in the initial workup of a bone tumor is the plain radiograph. CT and MRI are important to fully evaluate the extent of malignant bone tumor invasion for staging and treatment planning. Although bone tumors will be encountered frequently in daily practice, it is important to keep in mind that most are benign and can be managed conservatively.
References
- Gereige R, Kumar M. Bone lesions: Benign and malignant. Pediatr Rev. 2010; 31 (9): 355-362.
- Wyers MR. Evaluation of pediatric bone lesions. Pediatr Radiol. 2010; 40(4):468-473.
- Greenspan A, Remagen W. Differential Diagnosis of Tumors and Tumor-like Lesions of Bones and Joints. 1st ed. Philadelphia, PA: Lippincott-Raven, 1998.
- Khanna G, Bennett DL. Pediatric bone lesions: Beyond the plain radiographic evaluation. Semin Roentgenol. 2012; 47(1): 90-99.
- Greenspan A, Beltran J, Steinbach LS. Orthopedic Imaging - A Practical Approach. 6th ed. Philadelphia, PA: Wolters Kluwer Health, 2015.
- Kuntal AR, Meyer J, Ibrahim S, et al. The role of imaging of malignant bone tumors in children and young adults. Curr Probl Cancer. 2013; 37(4): 181-191.
- Rana RS, Wu JS, Eisenberg RL. Periosteal reaction. AJR Am J Radiol. 2009; 193(4): W259-W272.
- Dumitriu DI, Menten R, Clapuyt P. Pitfalls in the diagnosis of common benign bone tumours in children. Insights Imaging. 2014; 5(6): 645-655.
- Niu X, Xu H, Inwards CY, et al. Primary bone tumors. Epidemiologic comparison of 9200 patients treated at Beijing Ji Shui Tan Hospital, Beijing, China, with 10165 patients at Mayo Clinic, Rochester, Minnesota. Arch Pathol Lab Med. 2015; 139(9): 1149-1155.
Citation
A V, C M, CE B. Pediatric bone imaging: Differentiating benign lesions from malignant. Appl Radiol. 2018;(7):8-15.
July 6, 2018