Pleural Disease: A Review for the General Radiologist
Images

Pleural-related pathologies are frequently encountered on chest radiography and computed tomography (CT) studies. While general radiologists are likely familiar with entities such as pneumothoraces and simple pleural effusions, other pathologies may pose a diagnostic dilemma. We discuss three broad categories of pleural disease — neoplasia; infection and inflammation; and the unexpandable lung — with an emphasis on imaging features.
The pleura lines the thoracic cavity and comprises the visceral and parietal pleural layers. The inner visceral pleura covers the lungs and extends into the pulmonary fissures.1 The outer parietal pleura covers the mediastinum, diaphragm, and the innermost aspect of the chest wall. The potential space between the visceral and parietal pleura is the pleural space.
Pleural disease is most frequently encountered on chest radiography and CT, as these are routinely ordered in patients with signs and symptoms such as shortness of breath, cough, chest pain, or fever. Frontal and lateral chest radiographs are useful screening tools and can reveal many forms of pleural disease.
However, CT is the modality of choice for evaluating the pleura.1 In particular, a contrast-enhanced, thin-section CT of the chest with a scan delay of 60-90 seconds is optimal for assessing the visceral and parietal pleura, which together are normally less than 1 mm in thickness.2
Neoplasia
Metastasis
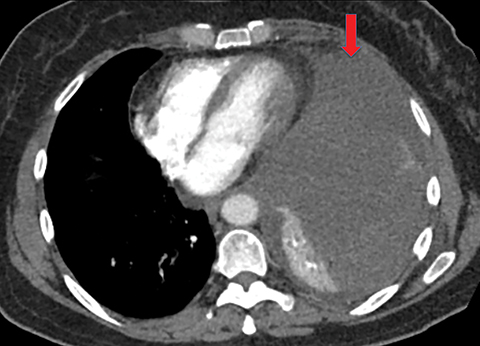
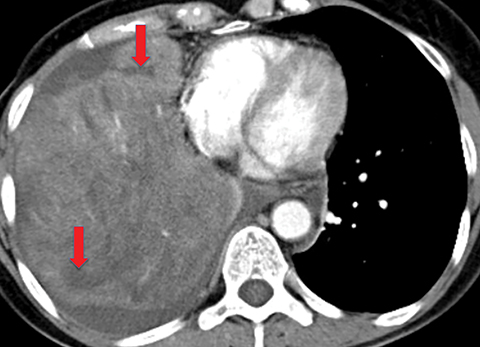
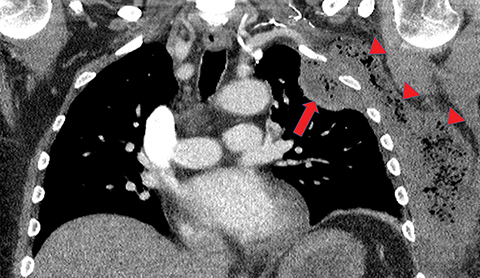
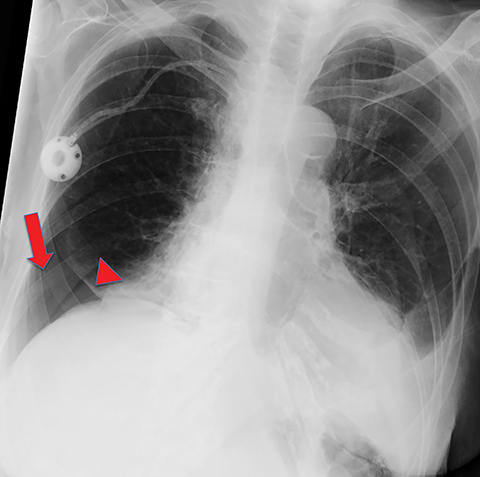
Metastasis is the most common neoplastic disease of the pleura; primary pleural tumors account for fewer than 5% of pleural neoplasms.3 Approximately 40% of pleural metastases are attributable to lung cancer, 20% to breast cancer, 10% to lymphoma, and 30% to various other primary malignancies.4 Pleural metastases may present as pleural effusions and should be included in the differential diagnosis of large, unilateral pleural effusions or loculated pleural effusions, especially in patients with a history of malignancy (Figure 1).
Such patients usually present with dyspnea and/or chest pain. The degree of dyspnea may not directly correlate to the volume of the effusion.5 Symptoms may relate to the patient’s baseline pulmonary status and the rate of fluid accumulation.
The diagnosis of malignant pleural effusion is most commonly established by thoracentesis, with subsequent pleural cytology. If a pleural biopsy is needed for diagnosis, positron emission tomography (PET) imaging or contrast-enhanced CT (CECT) may elucidate optimal targets for tissue sampling.
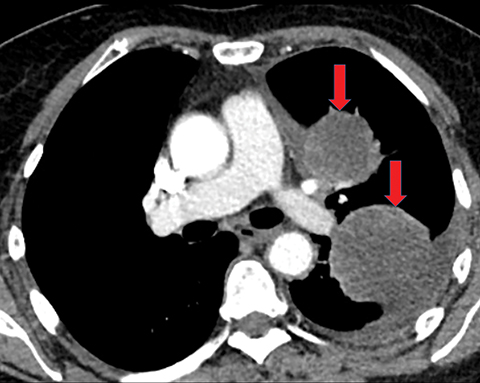
Pleural metastases can also manifest as pleural nodules and masses, which are also best evaluated with CECT (Figure 1). Findings concerning for pleural malignancy include circumferential pleural thickening, nodular pleural thickening, parietal pleural thickening greater than 1 cm, and involvement of the mediastinal pleura. In the presence of one or more of these findings, the sensitivity and specificity for pleural malignancy are 72% and 83%, respectively (Figure 2).6 These imaging features do not differentiate between pleural metastases and pleural mesothelioma.
Pleural metastatic disease indicates an advanced stage of cancer and generally a poor prognosis. Treatment is aimed at the goal of alleviating symptoms such as dyspnea and chest pain. Patients who are symptomatic from a large, malignant pleural effusion may benefit from indwelling tunneled pleural catheters or effusion drainage followed by pleurodesis.5,7,8 Patients with severe chest pain due to pleural metastases may benefit from blockade or lysis of the involved intercostal nerves.9,10
Mesothelioma
Mesothelioma, the most common primary pleural neoplasm, most often occurs in patients with a history of asbestos exposure. The malignancy has a very poor prognosis. Important as it is, a discussion of this complex malignancy is beyond the scope of this review. Interested readers are referred to articles dedicated to mesothelioma.11,12
Solitary Fibrous Tumor
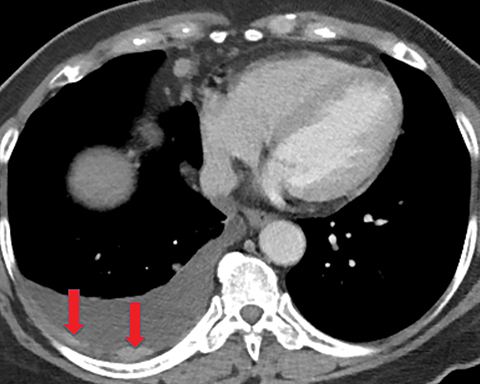
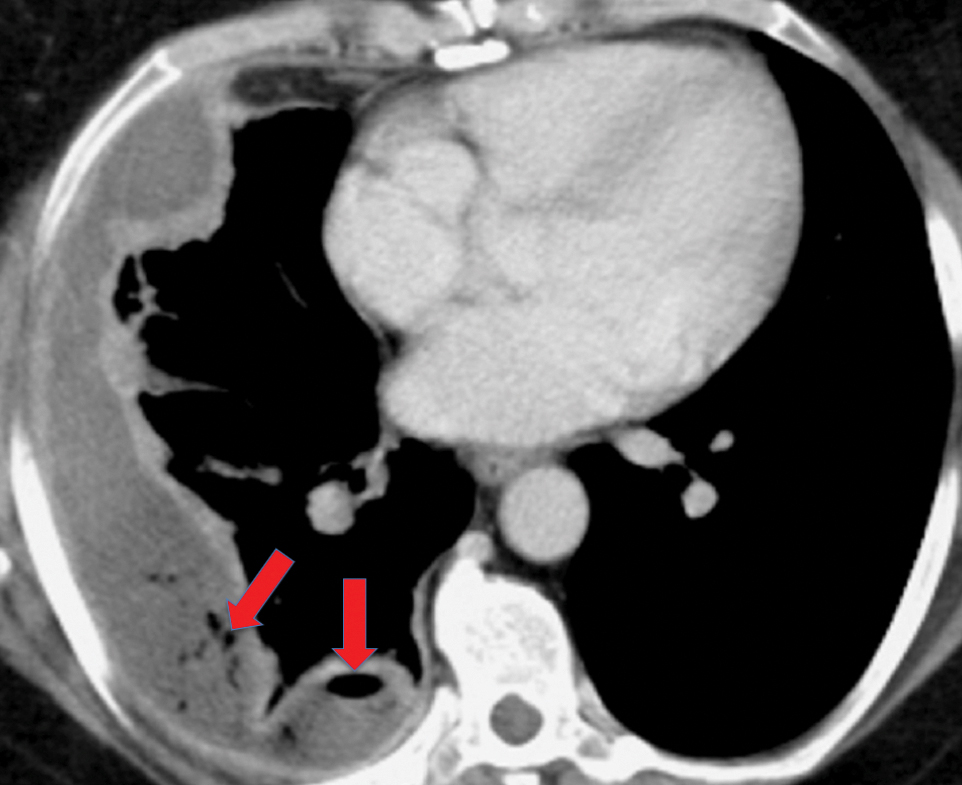
Solitary fibrous tumor of the pleura is a slow-growing tumor most commonly arising from the visceral pleura. Approximately 10-15% are malignant, and can metastasize widely.13 Patients are often asymptomatic, but some present with chest pain or dyspnea. Approximately 10% present with paraneoplastic syndromes, including those manifesting as hypoglycemia or hypertrophic pulmonary osteoarthropathy.13 Approximately half of solitary fibrous tumors are pedunculated and mobile, sometimes changing locations within the pleural cavity on imaging over time.1 They tend to be round with smooth margins. When still small, solitary fibrous tumors will typically enhance homogeneously, while larger tumors tend to enhance heterogeneously due to areas of necrosis (Figure 3).
Complete surgical resection is the treatment for solitary fibrous tumors. Attention to the resection site is recommended on follow-up imaging, as these tumors can be locally aggressive, regardless of their malignant potential.
Infection and Inflammation
Parapneumonic Effusion
Radiologists most frequently encounter pleural infection in the clinical setting of pneumonia. Approximately 50% of patients with pneumonia have a parapneumonic effusion, and roughly 10% of these effusions become infected; however, this complication carries a 20% mortality rate.14,15 Diagnosis requires thoracentesis and analysis of the pleural fluid. The infected fluid will be an exudate as determined by the Light criteria, with elevated pleural fluid protein or elevated pleural fluid lactate dehydrogenase (LDH) relative to serum protein or serum LDH levels. Fluid culture may reveal the pathogen, although cultures may be negative in as many as 40% of cases.16
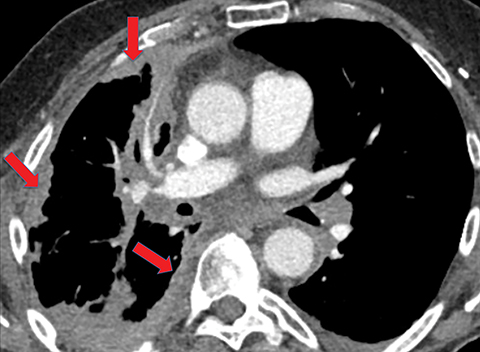
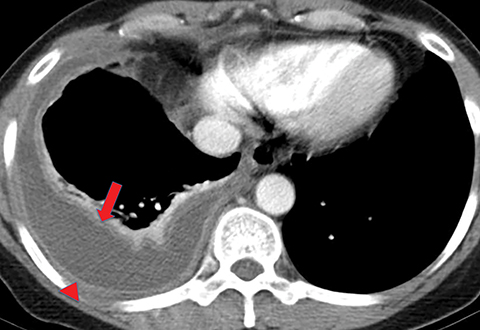
On imaging, complicated parapneumonic effusions are often loculated, owing to the fibrinous nature of the infected fluid (Figure 4). These effusions do not layer along the dependent portion of the pleural space as a simple effusion would. They tend to extend along nondependent aspects of the pleural space and into fissures.
Empyema
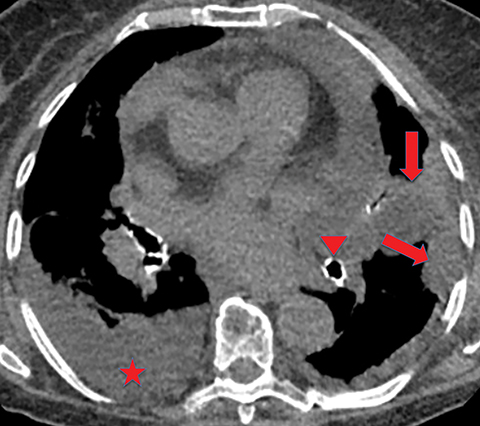
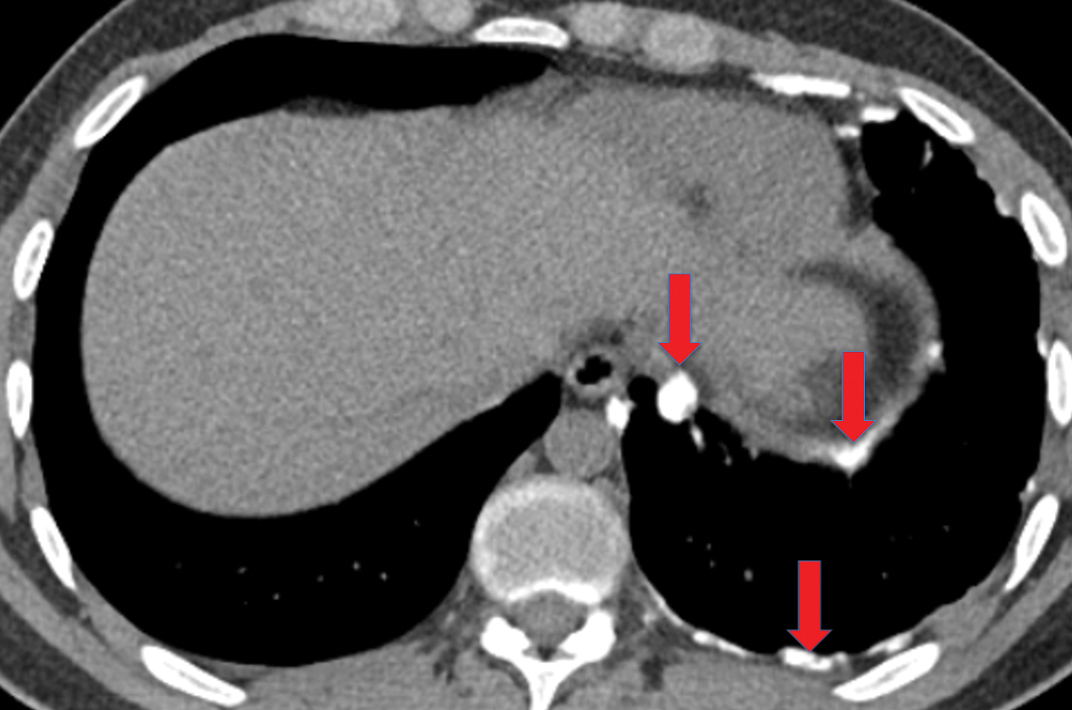
If the infection progresses, the effusion becomes an empyema, which is frankly purulent and viscous. There is thickening and enhancement of the visceral and parietal pleura on CECT, with infected fluid between these surfaces. This finding is called the “split-pleura sign”, and while it can be seen in the setting of other exudative pleural effusions, it is highly suggestive of empyema in the appropriate clinical setting (Figure 5). The absence of the split-pleura sign does not exclude a diagnosis of empyema. Gas within the pleural fluid is also highly suggestive of empyema. Gas-containing empyemas may indicate formation of a bronchopleural fistula; in such cases, thin-section CT images should be carefully reviewed for airways connected to the infected pleural infected (Figure 6).4
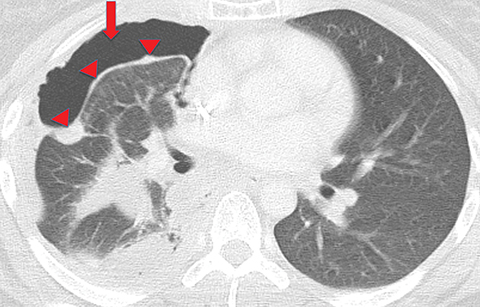
Extension of the infection beyond the parietal pleura and into the thoracic wall is termed empyema necessitans (Figure 7). Tuberculosis is the cause of empyema necessitans in about 70% of cases.17
Differentiating an empyema from a lung abscess may sometimes pose a challenge. An empyema tends to have smooth margins, exert mass effect on the lung with displacement of the lung parenchyma and airways, and is usually oblong. There may be associated stranding of the extrapleural fat. In contrast, a lung abscess has irregular or thick margins, is located within the lung parenchyma with possible necrosis of the parenchyma and airways, and is typically round. Both entities may contain gas.
Treatment of Parapneumonic Effusion and Empyema
Pleural infection treatment includes systemic antibiotics and evacuation of the infected pleural space, typically by large-bore chest tube(s). If the fluid is difficult to drain due to loculations or debris, then intrapleural fibrinolytics may be considered. The MIST2 trial concluded that the concurrent intrapleural administration of tissue plasminogen activator and DNase improved fluid drainage.18 However, a subsequent meta-analysis concluded that insufficient evidence exists to support the routine use of intrapleural fibrinolytics in patients with complicated pleural effusions and empyemas.19 If these treatments are ineffective, surgical intervention, such as decortication (removal of the fibrous cortex that forms on the infected pleura), is the next step in management.
Noninfectious Pleuritis
Pleural inflammation can occur in settings other than infection. These include hemothorax, post-cardiac surgery, radiation, uremic pleuritis, rheumatoid pleuritis, and chylous effusion. Chronic or remote inflammation of the pleura can result in CT findings such as chronic pleural effusions, pleural calcifications, and permanent thickening of the visceral pleura as a fibrous peel overlying the lung or permanent thickening of the parietal pleura (Figure 8). Reviewing prior imaging will demonstrate the chronic nature of these findings.
Unexpandable Lung
The term “unexpandable lung” refers to lung that cannot expand after pleural fluid drainage; the normal apposition of the visceral pleura and parietal pleura does not occur. It can manifest clinically as severe chest pain during thoracentesis or on imaging as a hydropneumothorax or pneumothorax ex vacuo after thoracentesis.20 One of the most common causes of unexpandable lung is the formation of a fibrous peel of the visceral pleura that prevents the lung from expanding. This peel can result from malignancy, infection, or noninfectious inflammation. The unexpandable lung is categorized as either an entrapped or a trapped lung.
Entrapped Lung
An unexpandable lung due to active pleural disease is referred to as an entrapped lung. The disease can be due to malignancy (either metastatic or primary), infection, or other inflammatory etiologies; it results in an exudative pleural effusion and a fibrinous visceral pleural peel. On pleural manometry, the presence of a pleural effusion causes slightly positive intrathoracic pressure. In a patient without entrapped lung, this pressure would slowly decrease to zero as pleural fluid is evacuated. However, in a patient with entrapped lung, the pressure would decrease to negative values during thoracentesis.20
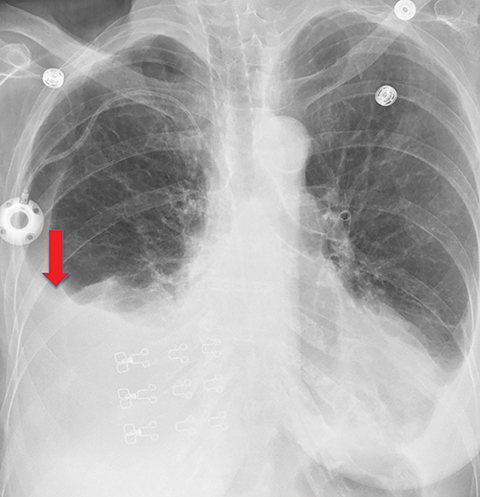
On imaging, patients with entrapped lung have pleural effusions (which may be loculated), or an empyema. When pleural malignancy is the underlying cause, pleural nodules or masses may be present. The thickened visceral pleural peel may be visible on CT (Figure 9). In patients with entrapped lung, imaging will show a hydropneumothorax or a pneumothorax ex vacuo when thoracentesis is performed. This occurs because the thickened visceral pleura covering the lung does not allow the lung to expand to fill the space previously occupied by the drained pleural fluid. The mechanism by which air enters the pleural space is unknown, but thought to be related to either needle placement or transient pressure-dependent pleuroparenchymal fistula21.
Treatment of entrapped lung focuses on its underlying cause, such as antibiotics and pleural fluid drainage in the setting of pleural infection, and indwelling pleural catheter in the case of malignant pleural effusion. Adequate treatment results in resolution of lung entrapment and normal apposition of the visceral and parietal pleura once the pleural fluid is drained. The goal is to prevent the development of a trapped lung.
Trapped Lung
An unexpandable lung resulting from chronic or remote pleural disease is referred to as a trapped lung. The chronic or remote pleural disease may be due to a variety of etiologies, all of which result in restrictive pleural fibrosis preventing the lung from expanding. Clinically, patients with trapped lung have a chronic transudative pleural effusion, and most are asymptomatic.20 On pleural manometry, these patients have an initial negative pleural pressure, which quickly becomes more negative as pleural fluid is drained.20
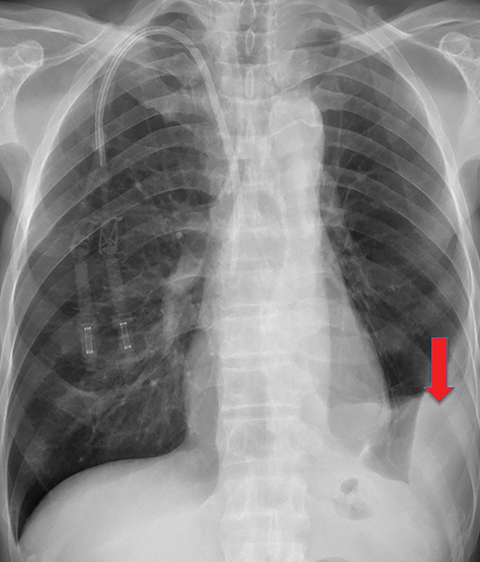
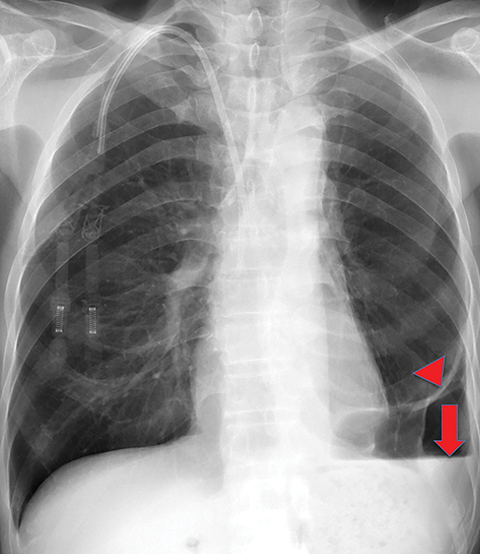
On imaging, trapped lung is accompanied by chronic pleural effusion(s). Prior imaging will show persistence of the effusion(s) over long periods of time, often years. It will also show longstanding pleural thickening and perhaps pleural calcifications, indicative of chronic or remote pleural disease. If thoracentesis has been performed or attempted, patients can develop a hydropneumothorax or pneumothorax ex vacuo due to the lung’s inability to expand (Figures 10, 11). Similar to the entrapped lung, CT of the trapped lung may also demonstrate thickening of the visceral pleura.
Symptomatic trapped lung is treated by placement of an indwelling pleural catheter. Pleurodesis is not performed because the procedure requires the apposition of the visceral and parietal pleura after iatrogenic irritation to induce fibrosis between the two inflamed pleural layers. Patients with trapped lung lack the apposition of the pleural layers; thus, pleurodesis is likely to fail.22
Conclusion
Pleural disease ranges from simple pleural effusions to malignant pleural tumors. Chest radiography and CT are commonly performed to evaluate patients presenting with signs and symptoms concerning for pulmonary or pleural disease. Therefore, radiologists interpreting these studies should be familiar with these broad categories of pleural disease and their management.
References
- Salahudeen HM, Hoey ET, Robertson RJ, et al. CT appearances of pleural tumours. Clin Radiol. 2009;64(9):918-930.
- Collins J, Stern E. Pleura, chest wall, and diaphragm. In: Chest Radiology: The Essentials. 3rd ed. Philadelphia, PA: Wolters Kluwer Health; 2015:169-201.
- Dynes MC, White EM, Fry WA, et al. Imaging manifestations of pleural tumors. Radiographics. 1992;12(6):1191-1201.
- Kuhlman JE, Singha NK. Complex disease of the pleural space: radiographic and CT evaluation. Radiographics. 1997;17(1):63-79.
- Walker S, Bibby AC, Maskell NA. Current best practice in the evaluation and management of malignant pleural effusions. Ther Adv Respir Dis. 2017;11(2):105-114.
- Leung AN, Muller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol. 1990;154(3):487-492.
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1):1800349 [https://doi.org/10.1183/13993003.00349-2018].
- Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318(19):1903-1912.
- Bhatnagar S, Gupta M. Evidence-based clinical practice guidelines for interventional pain management in cancer pain. Indian J Palliat Care. 2015;21(2): 137-147.
- Gulati A, Shah R, Puttanniah V, et al. A retrospective review and treatment paradigm of interventional therapies for patients suffering from intractable thoracic chest wall pain in the oncologic population. Pain Med. 2015;16(4): 802-810.
- Nickell LT, Lichtenberger JP, Khorashadi L, et al. Multimodality imaging for characterization, classification, and staging of malignant pleural mesothelioma. Radiographics. 2014;34(6): 1692-1706.
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics. 2004;24(1): 105-119.
- You X, Sun X, Yang C, et al. CT diagnosis and differentiation of benign and malignant varieties of solitary fibrous tumor of the pleura. Medicine (Baltimore). 2017;96(49): e9058. doi: 10.1097/MD.0000000000009058.
- Corcoran JP, Wrightson JM, Belcher E, et al. Pleural infection: past, present, and future directions. Lancet Respir Med. 2015;3(7):563-577.
- Perikleous P, Rathinam S, Waller DA. VATS and open chest surgery in diagnosis and treatment of benign pleural diseases. J Vis Surg. 2017; 3:84. doi: 10.21037/jovs.2017.05.03.
- Ferreiro L, Porcel JM, Bielsa S, et al. Management of pleural infections. Expert Rev Respir Med. 2018;12(6):521-535.
- Bandaru S, Manthri S, Sundareshan V, et al. Empyema necessitans in the setting of methicillin-susceptible Staphylococcus aureus causing pneumonia and bacteremia. Case Rep Infect Dis. 2018; doi:10.1155/2018/4906547.
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365(6):518-526.
- Janda S, Swiston J. Intrapleural fibrinolytic therapy for treatment of adult parapneumonic effusions and empyemas: a systemic review and meta-analysis. Chest. 2012;142(2):401-411.
- Pereyra MF, Ferreiro L, Valdes L. Unexpandable lung. Arch Bronconeumol. 2013;49(2):63-69.
- Heidecker J, Huggins JT, Sahn SA, et al. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-84.
- Bertolaccini L, Viti A, Paiano S, et al. Indwelling pleural catheters: a clinical option in trapped lung. Thorac Surg Clin. 2017;27(1):47-55.
Citation
SJ K, Lee A, WH M. Pleural Disease: A Review for the General Radiologist. Appl Radiol. 2020;(6):17-22.
November 6, 2020