Pregnancy-associated Breast Cancer and Other Breast Disease: A Radiologic Review
Images
Breast cancer is the most common malignancy affecting women, accounting for 30% of all new cancer diagnoses in 2018. Despite its high incidence, breast cancer carries a favorable prognosis, with a 90% five-year survival rate for all stages combined. 1 Improvements in screening techniques with advancement in both patient and clinician education have been paramount in detecting breast cancer at earlier stages and dramatically reducing overall mortality and morbidity.2, 3
A subset of breast cancer cases, specifically during pregnancy, is usually diagnosed at more advanced stages and carries a worse prognosis.4-6 Mortality rates were shown to be nearly 50% higher for cases of pregnancy associated breast cancer (PABC) compared with non-PABC.7 A meta-analysis of 3268 cases showed that PABC had a significantly higher risk of death than non-PABC with hazard ratio of 1.44; 95% CR [1.27-1.63]; PABC was independently associated with higher mortality, particularly those diagnosed shortly postpartum.8
Fortunately, PABC is relatively uncommon with an approximate incidence of 1 in every 3,000 pregnancies, comprising only 3% of all breast cancer cases.9,10 However, a recent large US population-based study showed an uptrend in the overall incidence of PABC between 2001-2013, which has been postulated to largely result from women delaying childbearing until a later age.10
PABC is defined as breast cancer presenting during pregnancy or up to one year postpartum. Naturally, PABC has added repercussions and diagnostic urgency given that the disease process and treatments will ultimately affect both the mother and fetus if diagnosed during pregnancy. Additionally, a myriad of physiological breast changes occur in response to the hormonal stimulation of pregnancy, which makes clinical and radiologic evaluation technically difficult.
Currently, there is a paucity of literature on PABC. Intrinsic challenges and lack of awareness have been postulated to contribute to the delay in diagnosis, which is a major factor in the overall poor prognosis.11 Thus, it is critical to be aware of this diagnosis. The goal of this article is to illustrate the expected changes to the breast during pregnancy and lactation and the appropriate diagnostic approach when evaluating pregnancy associated breast complaints. Also, this work highlights key imaging characteristics that can help distinguish benign from malignant processes.
Physiological Changes
During pregnancy and lactation, the breast undergoes physiological changes that are driven by fluctuating levels of estrogen, progesterone, and prolactin. Increasing estrogen levels secreted by the placenta induce growth of both the ductal system and surrounding stroma with large quantities of adipose tissue deposition and increased glandular vascularity. Simultaneously, increasing progesterone levels play a synergistic role with estrogen by causing further maturation of the breast lobules and alveoli with the secretory characteristics of the alveoli cells being developed.11-13
Estrogen and progesterone, though essential for breast development during pregnancy, cause inhibition of milk production. Concentrations of both these hormones significantly drop near the end of pregnancy and immediately after parturition, eliminating their inhibitory effects. On the other hand, secretion of prolactin by the pituitary gland steadily increases throughout pregnancy and, when unopposed by estrogen and progesterone, promotes the synthesis of milk. A few weeks postpartum, prolactin concentrations also return to basal levels; however, milk production is sustained by breast feeding, which induces intermittent spikes of marked prolactin secretion.
Clinically, these changes cause increasing firmness, volume, and nodularity to the breast which persists through lactation and makes physical examination progressively more problematic.11,14 This correlates with markedly increased fibroglandular breast parenchyma, which affects the appearance of the breast tissue on mammography, ultrasound, and MRI, and changes the overall sensitivity and utility of the various radiologic modalities.
Radiologic Evaluation
Most pregnant women fall below the recommended age for annual mammographic screening, with the median age at diagnosis of PABC being 33-34 years.4, 5 Thus, imaging evaluation of PABC is predominantly diagnostic in nature. Most patients present with a palpable breast lump that can easily be assessed with ultrasound (US). Furthermore, given the lack of radiation and high sensitivity of between 86.7% -100%, US is easily the test of choice.15,16
Sonographically, the increase in the non-glandular fibrofatty tissue during pregnancy causes the breast parenchyma to appear diffusely more hypoechoic.16-18 During lactation, the breast parenchyma becomes diffusely echogenic due to proliferation of glandular components and production of milk rich in fat. During this time, prominent ducts and increased vascularity can also be observed.14-18
Mammography may also be used to assess for PABC with physiological changes manifesting as marked increase in parenchymal density and breast size. 12 However, owing to the median age of women with PABC, mammography is typically recommended only if there is high suspicion for malignancy or to determine extent of disease.11 This is because mammography is less sensitive compared to ultrasound with studies reporting sensitivities between 78-87% in the setting of PABC.6, 15, 19 For lactating women 40 and older, mammography is considered appropriate.
Mammography is generally considered safe during pregnancy and lactation.20 It is important that radiologists be prepared to discuss radiation safety with patients and referring providers, who are often hesitant due to perceived risks. The fetus is most susceptible to radiation-induced malformations during the first trimester, believed to occur with exposure greater than 0.05 Gy of radiation. However, performing standard two-view mammography of each breast with abdominal shielding exposes the fetus to ~0.003-0.004 Gy, a minimal fetal radiation exposure. There is no proven carcinogenic effect of mammography in lactating women.11, 20
Currently, dynamic contrast enhanced (DCE) breast MRI is not recommended during pregnancy. Based on the current American College of Radiology (ACR) appropriateness guidelines, there is insufficient safety data for the fetus, and its diagnostic role has not been established. MRI is only recommended during pregnancy when the risk-benefit ratio is clearly defined by the disease process and predicted outcome.18, 20
During lactation, DCE breast MRI may be safely performed to evaluate the extent of disease or for high-risk screening. Gadolinium is considered safe during lactation with very low concentrations found in the produced milk.21 The current ACR guidelines do not require patients to discontinue breast feeding, though patients may pump and discard breast milk for 24 hours after gadolinium administration to fully excrete the contrast and thus avoid any ingestion by the infant.20,21
Since breast MRI is not typically performed during pregnancy, the physiological changes observed on MRI are typically of lactating women. Like other modalities, the physiological changes are manifested as increased breast parenchymal. A few small-scale studies have shown rapid, moderate to marked increased background enhancement, which is postulated to be secondary to increased perfusion. Additionally, there is diffusely increased T2 signal from milk production within the breast tissue.22, 23
Previous reports have hypothesized that the increased background enhancement would limit the value of DCE breast MRI in pregnant and lactating patients; however, a small case series showed that it was able to accurately detect all breast cancers in five cases.23 Also, in a recent retrospective study, preoperative MRI was shown to be 98% sensitive at detecting tumors. More importantly, in 28% of those cases, MRI changed surgical management by showing greater extent of disease or pathologically proven larger tumor burden compared to ultrasound and mammography.24
Another recent small cohort study showed that noncontrast breast MRI using diffusion tensor imaging (DTI) could be used in adjunct with other modalities in detecting PABC, evaluating the symptomatic breast, and screening high risk women during pregnancy.25 These studies show that MRI may be of value in the management of PABC despite current recommendations and further research to explore these roles is necessary.
Common Benign Disorders
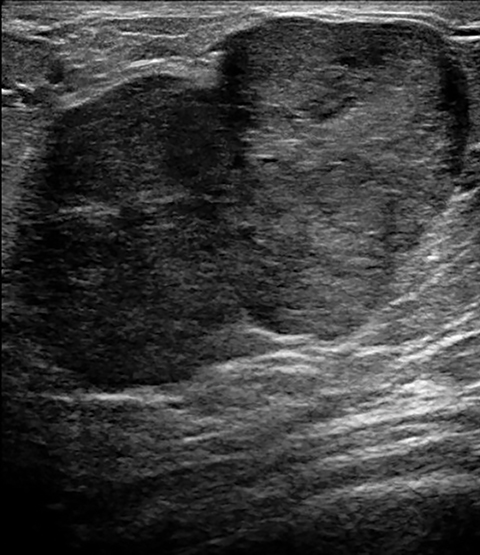
As in non-pregnant young females, the most common benign tumors associated with pregnancy are fibroadenomas. It is postulated that the majority of fibroadenomas are present before pregnancy but become apparent as they grow with rising hormone levels. Clinically, these present as painless, palpable, and rubbery masses, and are often multiple and bilateral. Imaging findings are similar to those of non-pregnant patients: an oval, well-circumscribed hypoechoic mass. In the setting of pregnancy or lactation, for a growing fibroadenoma or a new solid-appearing mass, even with benign features, image-guided biopsy and pathologic correlation is recommended (Figure 1).11, 13, 14
An uncommon benign entity that mainly occurs in the setting of pregnancy, typically in the 3rd trimester, is an infarcted fibroadenoma. The etiology is likely a rapidly growing fibroadenoma, secondary to increased hormones, that eventually outgrows its blood supply. This produces variable imaging patterns depending on the severity of infarction. These lesions can have heterogeneous echotexture, internal cystic spaces, and ill-defined or irregular borders, mimicking malignancy and often requiring pathological confirmation. Another form of fibroadenoma that may mimic malignancy is a fibroadenoma with secretory hyperplasia or lactational changes. This is thought to be due to hormone sensitive epithelial cells within the fibroadenoma, which are stimulated and undergo growth of the ductal elements in a similar manner to normal mammary tissue related to increased hormone levels. Sonographically, these typically show heterogeneous echotexture with hyperechoic areas, dilated ducts, and intralesional cysts. Secretory like calcifications may be seen on mammography.11, 13, 14
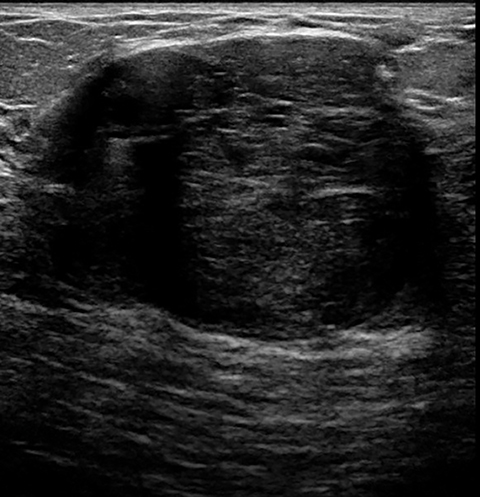
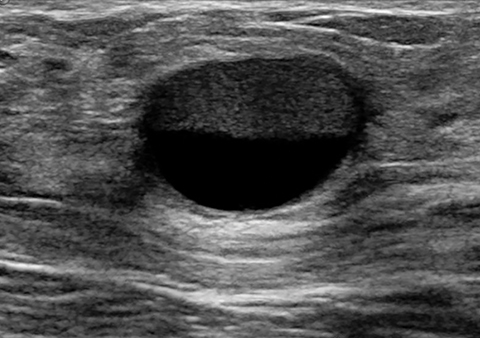
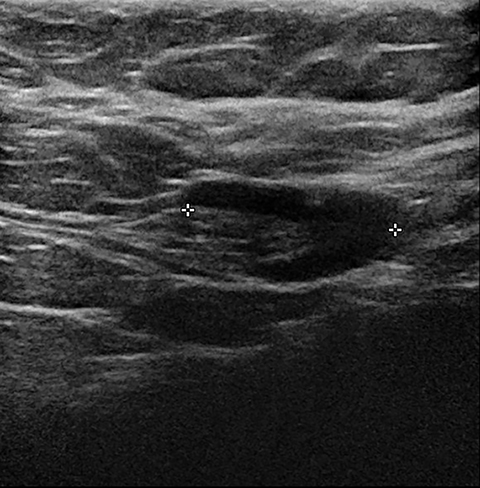
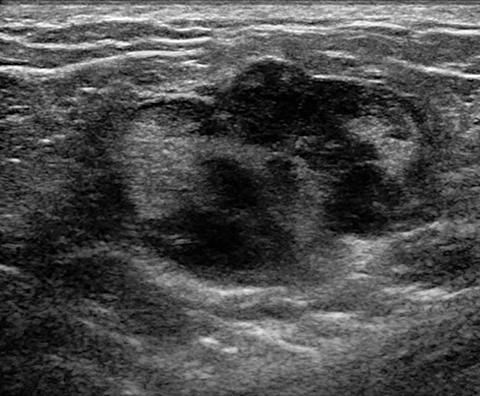
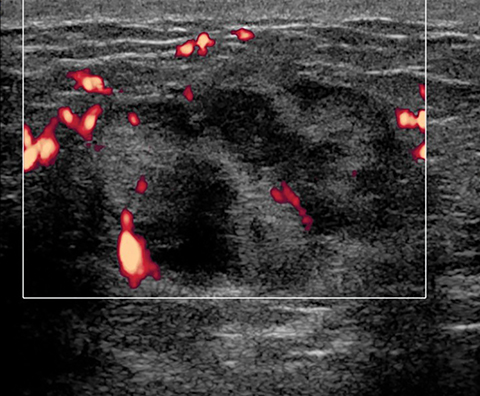
Lactating adenomas comprise a fourth kind of benign tumor and are unique to pregnancy and lactation. They are often indistinguishable from fibroadenomas on imaging and pathologically. They usually present as palpable, solid, and mobile masses during the 3rd trimester or during lactation and can be multiple and bilateral. Their typical sonographic appearance is of a solid hypoechoic and oval, mass that is circumscribed and may have microlobulations (Figure 2A); color Doppler evaluation typically shows internal vascularity (Figure 2B). Posterior acoustic enhancement may be present secondary to intralesional fluid. Like fibroadenomas, lactating adenomas may also infarct and be painful, causing the lesion to have a heterogeneous appearance under imaging which can mimic malignancy.13,14,26
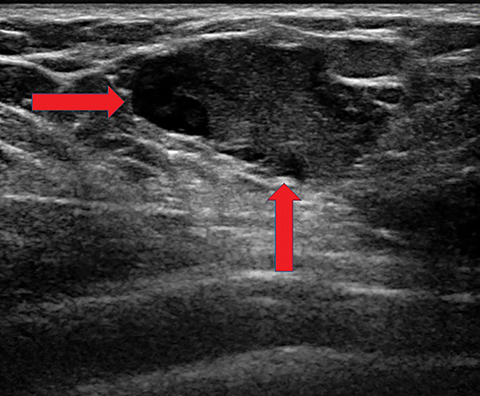
Lactating women can also develop galactoceles, which are the most common benign breast masses in these patients. Galactoceles occur predominantly after cessation of breastfeeding, but they occasionally can occur during lactation and, rarely, in the 3rd trimester.13 They are caused by milk obstructing a duct, inducing dilation of the obstructed portion and forming a complex cystic lesion. Fluctuating amounts of internal degraded milk products and surrounding inflammatory changes cause variable imaging appearances. Typically, these are oval shaped, with variable echogenicity depending on fat content and age of the lesion; they may begin as anechoic and show increasing echogenicity as they age. Internal vascularity should never be seen, although surrounding hyperemia is common due to surrounding inflammatory changes. Occasionally, a fat-fluid level may be observed that is pathognomonic (Figure 3). The majority of galactoceles resolve spontaneously.11, 13, 14
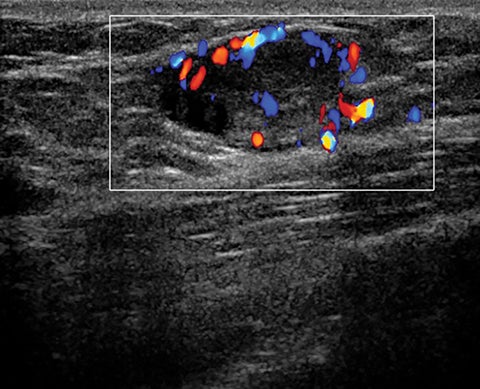
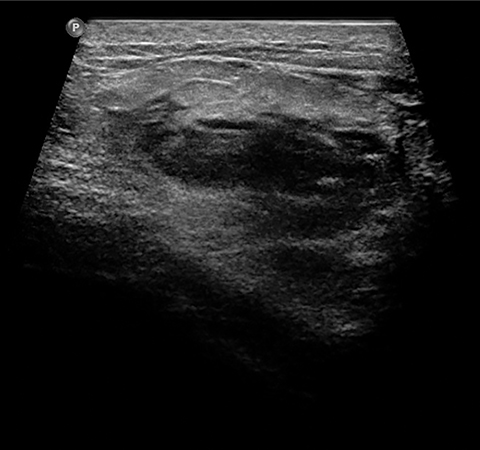
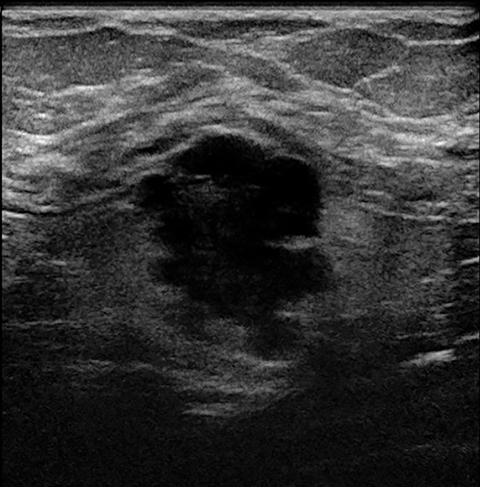
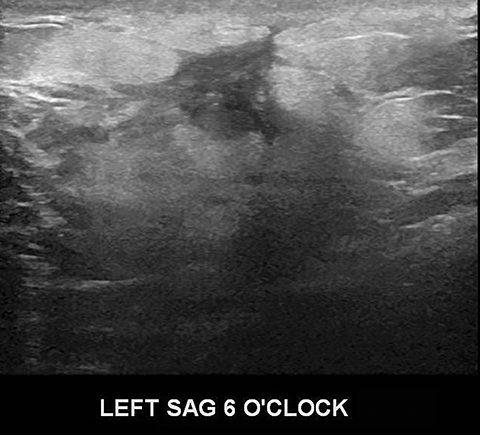
A fifth benign entity commonly seen during lactation but infrequently during pregnancy is puerperal mastitis. The mechanism of infection is disruption of the nipple epithelium and milk stasis, resulting in retrograde spread of bacteria, typically Staphylococcus aureus and Streptococcus. Patients clinically present with an erythematous, edematous, and painful breast. Diagnosis can be made based on clinical findings when uncomplicated, though imaging work-up may be necessary if there is poor response to antibiotics or concern of an underlying abscess. Ultrasound is the modality of choice, with sonographic findings of mastitis including skin thickening, decreased parenchymal echogenicity, and increased vascularity. An abscess will typically appear as a complex hypoechoic cystic mass with peripheral vascularity and posterior acoustic enhancement (Figure 4).14,16,18
Treatment of puerperal mastitis consists of antibiotics and frequent breastfeeding or breast expression to limit milk stasis. Ultrasound-guided aspiration should be considered for drainage of an abscess to provide pain relief and to shorten illness duration. If the abscess persists or recurs, repeat drainage should be performed.7,14,18 In atypical or refractory cases, further work-up with mammography and US should be considered, owing to the overlap of radiologic and clinical findings of infection and inflammatory breast cancer. Image-guided core needle biopsy or skin-punch biopsy may be necessary if there is suspicious imaging or clinical features.14,18
Pregnancy-associated Breast Cancer
Women with PABC typically present with a firm, painless, and palpable lump that is frequently non-mobile.15, 26 Additional presentations include unilateral breast enlargement with skin thickening, nipple retraction, and nipple discharge.27 As in non-pregnant patients, the most common breast cancer associated with pregnancy is invasive ductal carcinoma, with the pregnancy-associated malignancies having more aggressive histological features. Middleton et al showed that the most common histological subtype in 39 cases of PABC was a high-grade tumor that was estrogen and progesterone negative with increased rates of lymphovascular invasion.28
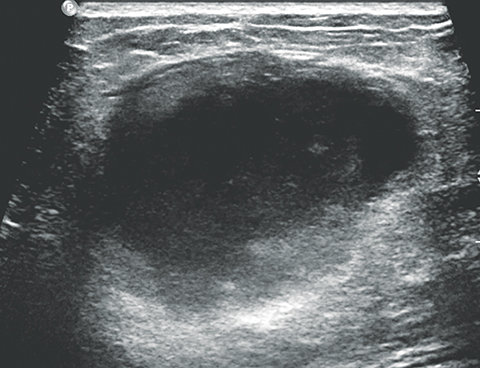
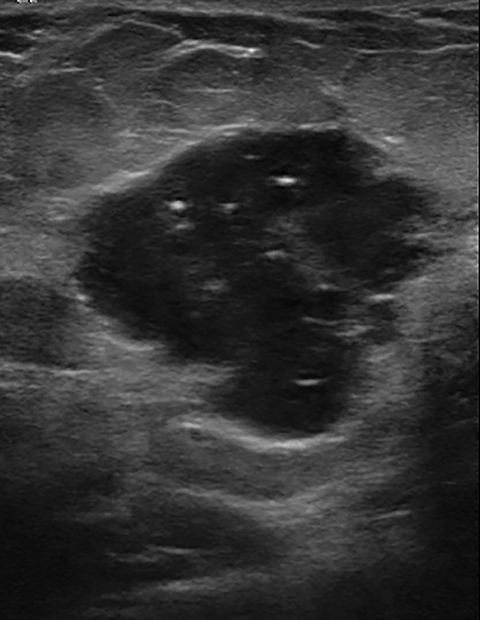
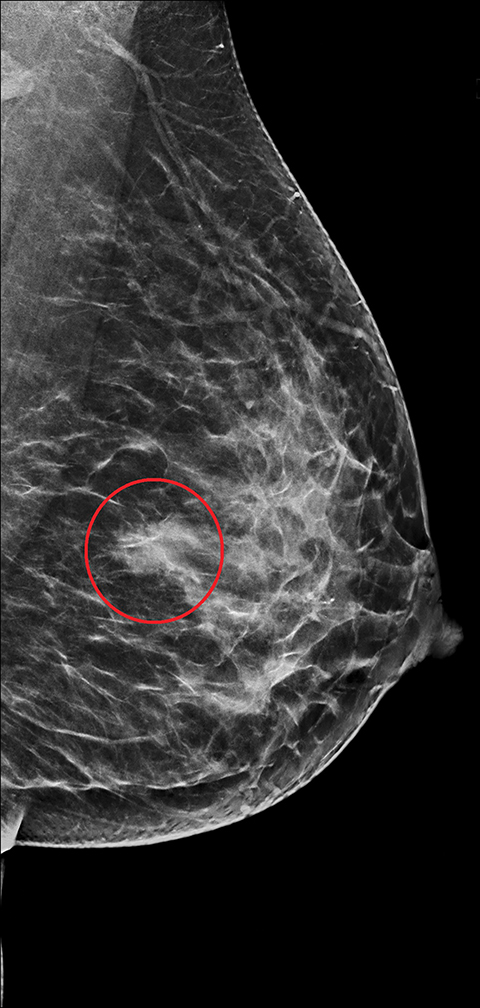
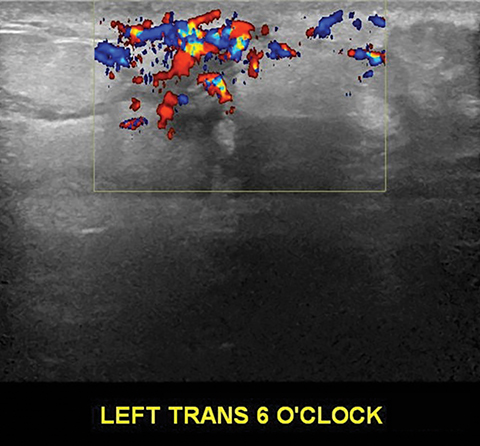
The imaging appearance of PABC is similar to that of non-gestational breast cancer. Nearly all cases of PABC present with the unifying sonographic finding of a mass.15, 27 Ultrasound features that typically signify a malignant rather than a benign process include a hypoechoic mass that is taller than it is wide, with indistinct or spiculated borders. Enlarged axillary lymph nodes may also be present in the setting of lymph node metastases. (Figures 5, 6) It has been reported that PABC often shows large cystic components, and up to 63% of these malignancies display posterior acoustic enhancement – findings that are typically associated with benign lesions.15, 23 Thus, it is imperative that biopsy be considered in the setting of a new solid mass found on ultrasound during pregnancy, even without suspicious features, to avoid delay in care.
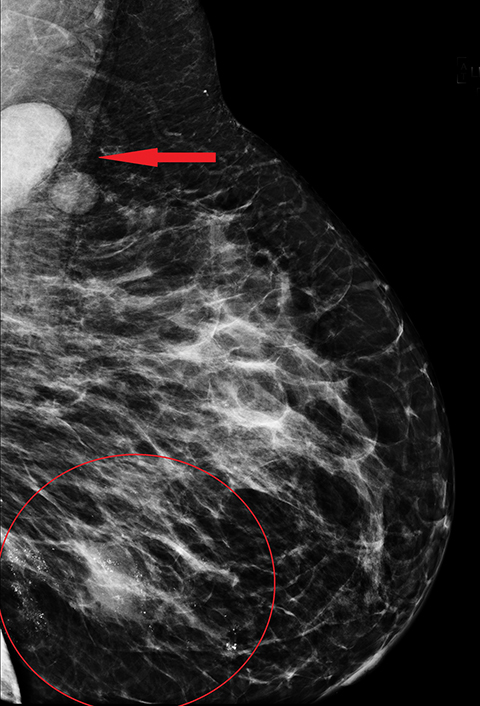
Mammographically, the most common presentation of PABC is a mass with or without associated features, such as skin thickening, calcifications, and axillary lymphadenopathy, followed by calcifications without an associated mass (Figures 5,6). A less common presentation is skin thickening with generalized increased parenchymal density, typically seen in inflammatory breast cancer. This presentation can be confused with puerperal mastitis, which can lead to a delay in diagnosis.6,15, 27
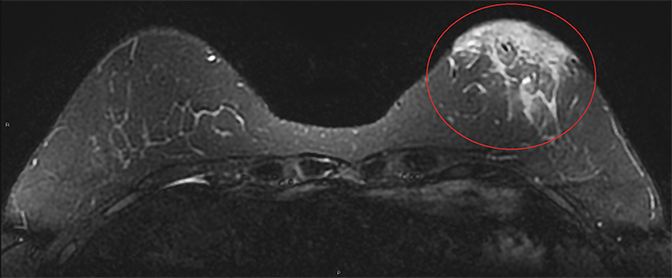
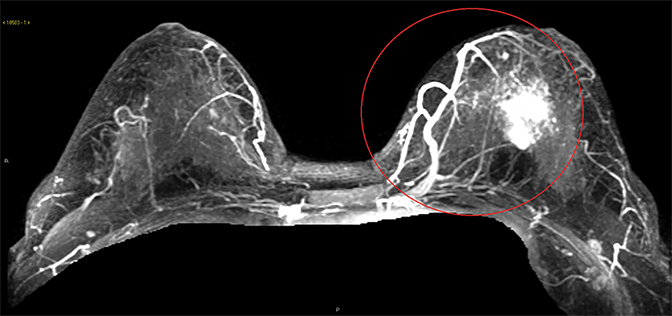
The literature on MRI evaluation of PABC is sparse, as contrast-enhanced MRI is considered contraindicated in pregnancy; most published research focuses on postpartum and lactating women. Despite concern that increased background enhancement could reduce sensitivity, a recent study by Myers et al showed that contrast-enhanced MRI had a sensitivity of 98% in detecting malignancy. The most common MRI appearance was of an enhancing mass with 62% of the PABC displaying an irregular shape and 66% having irregular margins.24 In a recent prospective cohort by Nissan et al evaluating the feasibility of noncontrast MRI using DTI, PABC most often appeared as decreased diffusivity and maximal anisotropy values when compared to normal pregnant fibroglandular tissue.25
Atypical Pregnancy-related Malignancies of the Breast
There are two distinct but uncommon types of primary lymphomas that affect the breast, both of which are especially rare during pregnancy and lactation. The first, Burkitt Lymphoma, is the less common of the two, presenting as diffuse bilateral breast enlargement that affects younger puerperal women. Classically, this type of breast lymphoma is categorized as the endemic or African type, which has been linked to the Epstein-Barr virus, though there are also sporadic cases. On imaging, this malignancy manifests as diffuse bilateral increase in parenchymal density corresponding to the infiltrative tumor process.11, 29 The more common subtype, non-Hodgkin lymphoma of the breast, typically affects older women. These are usually unilateral with presentation and imaging features that are similar to those of breast carcinoma.29 Although this lymphoma is not typically associated with pregnancy, there are a few confirmed cases in puerperal women (Figure 7).
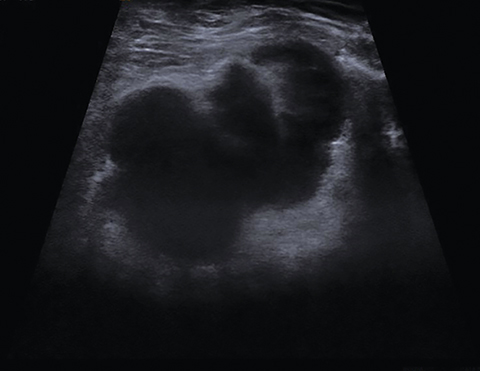
Another rare entity that can affect puerperal women is primary breast angiosarcoma. This aggressive tumor occurs sporadically in younger women; 6-12% of these tumors are associated with pregnancy or lactation.30 Clinically, they present as a painless, rapidly growing, and palpable mass, often accompanied by an adjacent bluish skin discoloration.30, 31 The mammographic appearance is nonspecific, with the most common presenting feature being an ill-defined noncalcified mass or a focal asymmetry. Up to 33% of breast angiosarcomas are mammographically occult. Sonographically, these tumors display varying echogenicity and may have circumscribed or ill-defined borders; on color Doppler, angiosarcomas typically display marked hypervascuarlity (Figure 8). On MRI, these tumors appear heterogeneous, displaying decreased signal intensity on T1 sequences and high signal on T2 sequences. Low-grade angiosarcomas typically show a persistent enhancement pattern on contrast-enhanced MRI, while high-grade tumors display rapid enhancement and washout, with large draining vessels possibly being visualized (Figure 8).31
Conclusion
PABC is a subset of breast cancer that carries a poor prognosis. During pregnancy, the breast undergoes many physiologic changes that make an imaging diagnosis challenging. Ultrasound is the primary imaging modality in the diagnostic work-up for PABC, though mammography and MRI may also play a role. Palpable masses presenting in pregnant or lactating women should undergo prompt imaging evaluation. New or growing solid masses should be further evaluated with biopsy to avoid delaying a potential cancer diagnosis.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68(1):7–30.
- Cortesi L, Chiuri VE, Ruscelli S, et al. Prognosis of screen-detected breast cancers: results of a population-based study. BMC Cancer. 2006; 6:17
- Tabár L, Dean PB, Chen TH, et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer. 2019; 125(4):515-523
- Ring AE, Smith IE, Ellis PA. Breast cancer and pregnancy. Ann Oncol. 2005; 16:1855–1860.
- Navrozoglou I, Vrekoussis T, Kontostolis E, et al. Breast cancer during pregnancy: a mini-review. Eur J Surg Oncol. 2008; 34:837–843.
- Liberman L, Giess CS, Dershaw DD, et al. Imaging of pregnancy-associated breast cancer. Radiology. 1994; 191:245–248.
- Johansson AL, Anderson TM, Hsieh CC, et al. Stage at diagnosis and mortality in women with pregnancy associated breast cancer (PABC). Breast Cancer Res Treat. 2013;139(1):183-92
- Azim HA Jr, Santoro L, Russel-Edu W, et al. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev. 2012; 38(7):834-42.
- Pavlidis N, Pentheroudakis G. The pregnant mother with breast cancer: diagnostic and therapeutic management. Cancer Treat Rev. 2005; 31:439–47.
- Cottreau CM, Dashevsky L, Andrade SE, et al. Pregnancy-Associated Cancer: A U.S. Population-Based Study. J Womens Health (Larchmt). 2019;28(2):250-257.
- Sabate JM, Clotet M, Torrubia S, et al. Radiologic evaluation of breast disorders related to pregnancy and lactation. Radiographics. 2007;27(Suppl 1): S101–S124.
- Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology, 13th ed. Philadelphia, PA: Saunders/Elsevier, 2016
- Stavros AT. Breast ultrasound. Philadelphia, PA: Lippincott Williams & Wilkins, 2004
- Vashi R, Hooley R, Butler R, et al. Breast imaging of the pregnant and lactating patient: physiologic changes and common benign entities. AJR Am J Roentgenol. 2013; 200:329–336.
- Ahn BY, Kim HH, Moon WK, et al. Pregnancy-and lactation-associated breast cancer: mammographic and sonographic findings. J Ultrasound Med. 2003; 22: 491–497.
- Holanda AAR, Gonçalves AKS, Medeiros RD, et al. Ultrasound findings of the physiological changes and most common breast diseases during pregnancy and lactation. Radiol Bras. 2016; 49:389–396.
- Ramsay DT, Kent JC, Hartmann RA, et al. Anatomy of the lactating human breast redefined with ultrasound imaging. J Anat. 2005; 206:525–534.
- Joshi S, Dialani V, Marotti J, et al. Breast disease in the pregnant and lactating patient: radiological-pathological correlation. Insights Imaging. 2013; 4:527–538.
- Yang W, Dryden M, Gwyn K, et al. Imaging of breast cancer diagnosed and treated with chemotherapy during pregnancy. Radiology. 2006; 239:52-60
- diFlorio-Alexander RM, Slanetz PJ, Moy L, et al. ACR Appropriateness Criteria® Breast Imaging of Pregnant and Lactating Women. Available at https://acsearch.acr.org/docs/3102382/Narrative/. American College of Radiology. Accessed Date 2/4/2019
- Webb JA, Thomsen HS, Morcos SK. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol.2005; 15: 1234–1240.
- Talele AC, Slanetz PJ, Edmister WB, et al. The lactating breast: MRI findings and literature review. Breast J. 2003; 9:237-240
- Espinosa LA, Daniel BL, Vidarsson L, et al. The lactating breast: contrast-enhanced MR imaging of normal tissue and cancer. Radiology. 2005; 237:429–436.
- Myers KS, Green LA, Lebron L, et al. Imaging appearance and clinical impact of preoperative breast MRI in pregnancy-associated breast cancer. AJR Am J Roentgenol. 2017; 209(3):W177-W183.
- Nissan N, Furman-Haran E, Allweis T, et al. Noncontrast breast MRI during pregnancy using diffusion tensor imaging: a feasibility study. J Magn Reason Imaging. 2019; 49(2): 508-517.
- Barco NI, Vidal MC, Fraile M, et al. Lactating adenoma of the breast. J Hum Lact. 2016; 32(3):559-62.
- Vashi R, Hooley R, Butler R, et al. Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. AJR AM J Roentgenol. 2013; 200(2):321-8.
- Middleton LP, Amin M, Gwyn K, et al. Breast cancer in pregnant women: assessment of clinicopathologic and immunohistochemical features. Cancer. 2003; 98:1055-1060.
- Bobrow LG, Richards MA, Happerfield LC, et al. Breast lymphomas: a clinicopathologic review. Hum Pathol. 1993; 24:274–278.
- Pramanik R, Gogia A, Malik PS, Gogi R. Metastatic primary angiosarcoma of the breast: can we tame it the metronomic way. Indian J Med Paediatr Oncol. 2017; 38:228–31.
- Glazebrook K.N., Magut M.J., Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008; 190:533–538.
Citation
A O, SC H, LA M.Pregnancy-associated Breast Cancer and Other Breast Disease: A Radiologic Review. Appl Radiol. 2020; (5):10-17.
September 1, 2020