ProFound AI Version 3.0 for DBT gets FDA Clearance
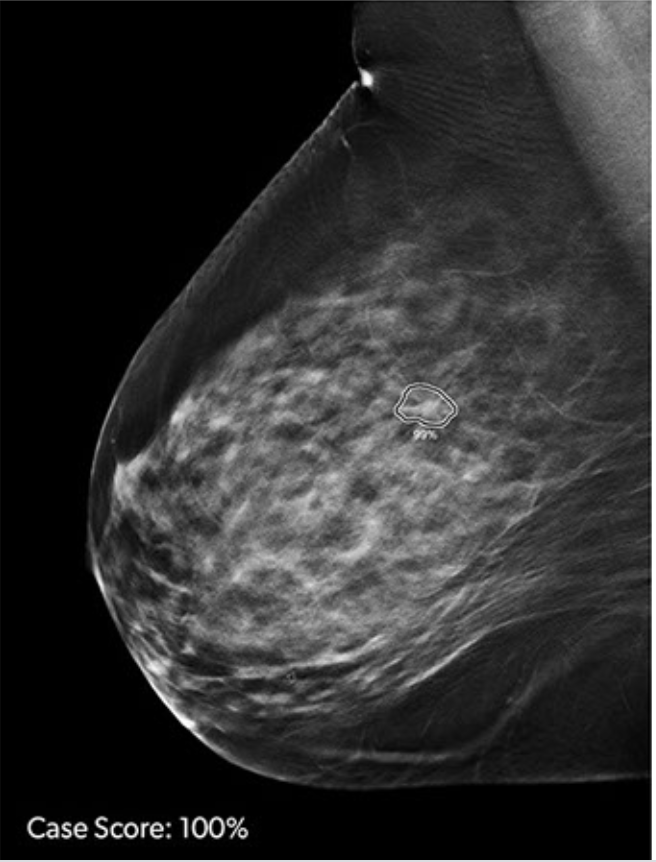 iCAD, Inc. announced US FDA clearance of ProFound AI Version 3.0 for digital breast tomosynthesis (DBT). ProFound AI for DBT is a high-performance, deep-learning, workflow solution trained to detect malignant soft tissue densities and calcifications. According to the company, the ProFound AI 3.0 algorithm delivers up to a 10% improvement in specificity performance and up to 1% improvement in sensitivity compared to the prior version. ProFound AI Version 3.0 also provides up to 40% faster processing on the company’s PowerLook platform.
iCAD, Inc. announced US FDA clearance of ProFound AI Version 3.0 for digital breast tomosynthesis (DBT). ProFound AI for DBT is a high-performance, deep-learning, workflow solution trained to detect malignant soft tissue densities and calcifications. According to the company, the ProFound AI 3.0 algorithm delivers up to a 10% improvement in specificity performance and up to 1% improvement in sensitivity compared to the prior version. ProFound AI Version 3.0 also provides up to 40% faster processing on the company’s PowerLook platform.
“The FDA Clearance of ProFound AI Version 3.0 is yet another milestone that positions iCAD and our technology as vanguards in the cancer detection realm. Our third generation AI solution for DBT may afford physicians the ability to interpret an increasing amount of data in DBT cases and analyze each image to detect malignant lesions more efficiently and with even greater precision,” said Michael Klein, Chairman and CEO of iCAD. “Improvements in specificity, which correlates with reductions in false positives, typically come at the expense of sensitivity and cancer detection scores. To increase sensitivity while simultaneously improving specificity is a huge performance achievement.”
Built with deep-learning technology, ProFound AI for DBT rapidly analyzes each tomosynthesis image, detecting malignant soft tissue densities. Certainty of Finding and Case Scores are relative scores computed by the ProFound AI algorithm and represent its confidence that a detection or case is malignant. The Certainty of Finding scores help radiologists by aiding in clinical decision making. Case Scores, which are assigned to each case by the algorithm, help clinicians to gain a sense of case complexity, which may be useful for prioritizing the reading work list.
ProFound AI Version 3.0 was developed using over five million images from 30,000 cases, including almost 8,000 biopsy-proven cancers, and validated on approximately one million images from 3,500 cases that included 1,200 biopsy-proven cancers.