The diagnostic role of magnetic resonance enterography in Crohn’s disease: An updated review of techniques, interpretation, and
Images





Crohn’s disease (CD), a chronic illness characterized by transmural involvement of the bowel wall, mainly affects young adults with a relapsing and remitting course. Clinical presentation typically involves nonspecific symptoms that can be associated with an array of acute active and chronic disease, as well as related to complications from primary bowel pathology that includes the extra-enteric tissues within the abdomen or pelvis. An optimal imaging technique can noninvasively assess the bowel wall for changes in active or chronic CD, can identify complications related to transmural CD, and can be used to monitor the disease safely with minimal or no X-ray exposure.
Crohn’s disease involves the small bowel in 80% of cases and can be challenging to diagnose and monitor. In addition, CD involves the full thickness of bowel wall; submucosal inflammation is responsible for tissue destruction and penetration, leading to such complications as fibrosis, strictures, and fistulae. These processes are responsible for the most serious morbidities whereas, in contrast, disease restricted to mucosa is not associated with such sequelae.
While endoscopy with biopsy is generally considered the diagnostic reference standard, this combination evaluates only the mucosa; it does not assess inflammation or fibrosis within the submucosa or deeper tissues. Therefore, these optical techniques alone may under-represent the extent of disease, particularly when considering that the mucosa has a high capacity for repair.
The overarching objective of imaging is to diagnose CD, to assess the location, mural and extramural extent and severity of disease, and to assess for complications such as stricture, bowel tethering, obstruction, or abscess. Cross-sectional imaging, unlike optical techniques, can evaluate submucosal and deeper tissues and extraintestinal complications of CD. Currently, computed tomography enterography (CTE) and magnetic resonance enterography (MRE) are the only 2 imaging modalities that can visualize submucosal tissues throughout the small bowel. CT generally is highly utilized,1 but there is growing concern over ionizing radiation and cancer risk. In contrast to CTE, MRE does not expose patients to ionizing radiation, and it can differentiate between inflammation and fibrosis as the cause of submucosal inflammation (Table 1). These advantages may be used to monitor the effects of medical therapy more accurately, to detect residual active disease even in patients showing apparent clinical resolution,2 and to triage patients for surgical intervention more accurately.
Review of diagnostic methods
Clinical subjective disease activity assessment
Crohn’s disease activity measurements, including the Physician Global Assessment, the Harvey-Bradshaw Index, the Crohn’s Disease Activity Index (CDAI) and the Pediatric Crohn’s Disease Activity Index (PCDAI), are predominantly subjective measurements.3-5 While multiple studies have demonstrated no correlation between MRE (Magnetic Resonance Enterography) findings and the CDAI,6-9 other studies have shown such a correlation10-12 as well as a correlation between MRE and laboratory markers of inflammation ESR/CRP (erythrocyte sedimentation index/c-reactive protein).3-5 Only 2 pediatric studies have compared MRE to PCDAI; one demonstrated a statistically significant correlation between disease on MRE and PCDAI,13 and the other demonstrated no correlation between MRE and pediatric CDAI.14 Subjective clinical activity measurements do not essentially reflect mucosal findings, with these studies showing discordance between inflammation on endoscopy and subjective activity index measurements. This suggests intrinsic unreliability of subjective techniques for assessment of bowel wall and extra-enteric soft tissue pathology.
Endoscopic techniques
Ileocolonoscopy directly visualizes the mucosa and facilitates tissue sampling, providing high diagnostic sensitivity for mucosal disease. However, only a short segment of terminal ileum may be accessible with this technique. Video capsule endoscopy (VCE) visualizes the mucosa throughout the small bowel, but does not provide tissue samples and is contraindicated in suspected bowel stenosis and obstruction. Endoscopic methods, even with biopsy, evaluate only the extent of mucosal disease; submucosal and serosal-mesenteric disease will not be fully appreciated.15 Three studies, each with approximately 20 patients, have compared MRE to VCE. They concluded that MRE and VCE identified diseased small bowel; however, VCE identified more lesions and small aphthous lesions,16-18 reflecting the authors’ contention that MRE is relatively less sensitive to early, mild disease restricted to the mucosa.
Small-bowel follow through
Small-bowel follow-through (SBFT) has been developed to evaluate the small bowel and to detect mucosal disease (aphthous ulcers), and complications, such as stricture, fistula, and abscess. However, SBFT is an x-ray-based technique that is relatively insensitive to mucosal disease and provides limited sensitivity for submucosal or deeper disease involvement. Both CTE and MRI have been shown superior to SBFT,19 particularly for detecting extra-enteric disease and complications.
CTE
The advantages of CTE over MRE include greater availability and lower initial costs, although the overall cost benefit is a key measure that remains incompletely evaluated. Some studies comparing MRE to CT in small-bowel pathology have indicated similar sensitivities, while 1 has indicated19,20 better sensitivity for CT,21 and 1 other study has indicated better sensitivity for MRE.22 The apparent wide range of results with MRE relative to CTE is largely due to the wide range of sequence techniques implemented. The authors contend that MRE accuracy will be limited if optimized, fat-suppressed, single-shot, T2-weighted acquisitions, such as the spectral adiabatic single-shot, fast spin-echo method, which have been previously described, are omitted.23,24 These techniques are discussed later in this review.
A significant limitation of CTE is concern over cumulative ionizing radiation dose in patients who may otherwise benefit from longitudinal imaging evaluation over the course of their disease. As a relative comparison, it has been shown that one CTE exam can lead to 5 times the organ effective dose of a SBFT exam.25
Primary diagnostic role of MRE
MRE may more accurately depict submucosal pathology in transmural CD, including determining the presence and extent of inflammation, fibrotic disease, and other intra-abdominal complications, compared to other diagnostic modalities, including CT (Table 1). Further evidence supports that another diagnostic strength of MRE is the ability to differentiate inflammation from fibrosis within the submucosa of the bowel wall and within the peri-enteric tissues (Table 1 and 2).15,22,23 MRE can show enteric as well as extra-enteric complications, including bowel obstruction, abscesses, tethering, and fistulae.26-29 These manifestations may be visualized on MRE with less dependency on bowel distension as required for optimal CT imaging, an important technical advantage of MRE.30
Technical review of MRE
MRE initially was designed to simulate the concept of luminography, as established for SBFT, by obtaining images that resemble fluoroscopic small-bowel images. This was achieved by administering a large volume of fluid either by mouth or by naso-jejunal tube (MR enteroclysis). MR images were acquired using thick-section (5-8 cm), single-shot, echo-train, spin-echo images strongly T2-weighted (T2W) to obtain images similar to those of SBFT. Distending the small bowel with water-based methods offers a bright luminal signal on T2W, and dark signal on T1-weighted (T1W) techniques. To maintain adequate distension, a viscosity agent may be added or osmotic agents like 2.5% mannitol, a nondigested carbohydrate, can be used to slow water absorption. Distension for MR luminography is achieved by administering at least 1 liter of water-based contrast by mouth 20 min to 30 min prior to the examination. This can also be accompanied by intravenous metoclopramide or erythromycin to promote gastric emptying.31, 32 To improve image quality on motion-sensitive T1W 3D GRE sequences, 1 mg of glucagon may be administered intravenously to reduce artifacts from peristalsis.
Although both bright- and dark-lumen contrast agents have been suggested, water-based methods are relatively easy to implement and provide excellent signal characteristics, resulting in bright lumen on T2W and dark lumen on T1W techniques. To slow the absorption that normally would occur in the jejunum, osmotic and viscosity agents are added. In the early development of MRE, others proposed routinely using enteroclysis via a naso-jejunal tube to administer intra-luminal contrast for superior small-bowel distension.33
Optimized MR image acquisitions
A variety of T1W and T2W sequences are available, but the most useful sequences for bowel imaging include the breathhold 3-dimensional gradient echo (3D GRE) T1W and the single-shot T2W techniques.15 The latest generation 3D GRE has provided volumetric imaging with higher in-/out-of-plane resolution and contiguous bowel-segment imaging with improved contrast and edge sharpness. The multi-echo Dixon technique has provided further improvement in fat suppression. T1W images are acquired after gadolinium-based contrast is administered to selectively enhance diseased bowel wall. Fat surrounds the bowel and can interfere with visualizing disease; inflamed bowel and fat both produce high signal on T2W and gadolinium-enhanced T1W images. Fat-suppression techniques are critical to improve disease conspicuity. The diseased bowel generates high signal, which becomes highly conspicuous only if the adjacent fat is completely darkened by fat-suppression.24 T1W gadolinium-enhanced fat-suppressed and T2W, single-shot images, with and without fat-suppression, are the foundation of diagnosing and characterizing CD. Regular T2W single-shot images depict bowel-wall morphology. Fat-suppressed single shot T2W images are critical for assessment of edema and inflammation related to active CD. Technical improvements include T2W single-shot partial Fourier reconstruction coupled with parallel processing for echo-space compression, which leads to improved signal-to-noise, contrast and edge sharpness of bowel-wall morphology. Optimal suppression of fat is achieved using spectral adiabatic inversion recovery (SPAIR).23, 24
Optimized MRE patient preparation
Bowel distension may be achieved using typical oral contrast methods for routine CT examination of the abdomen. These water-based contrast agents contain an osmotic agent, preventing absorption, and a viscosity agent, improving small-bowel distension. The water content of these agents produces low signal within the lumen and generates contrast against the enhancing bowel wall on gadolinium-enhanced T1W fat-suppressed images. The water content produces high signal within the bowel lumen on T2W images against a normally low-signal bowel wall. CT and fluoroscopic techniques are usually nondiagnostic in assessing pathological bowel-wall thickening without marked bowel distension. The authors have found MRE sensitive to bowel-wall pathology related to CD even without any distension of the affected segments, a significant benefit of MRE.15 In symptomatic patients who are unable to take oral contrast, we proceed without delay to the MRE procedure and have found little adverse impact on diagnostic outcomes.
Spectrum of MR imaging features of CD
The imaging manifestations of CD shown by MRE are summarized in Table 2.
Common T1W imaging features include: 1) Bowel-wall thickening with increased enhancement on delayed images; 2) Stranding extending into the mesenteric border fat and increased size and number of vessels; 3) Accordion-like compression and thickening of folds asymmetrically involving the mesenteric side of the small bowel having a tethered appearance; and 4) Reactive enlarged adjacent mesenteric nodes.
Common T2W imaging features include: 1) Bowel-wall thickening with increased signal in and adjacent to the abnormal bowel (on fat-suppressed images) showing active inflammation; and 2) Fluid accumulation in adjacent intra-peritoneal and mesenteric spaces.
Interpretive approach to a thickened bowel wall segment: Imaging interpretation of bowel-wall thickening is the cornerstone of CD diagnosis required for therapeutic decisions.
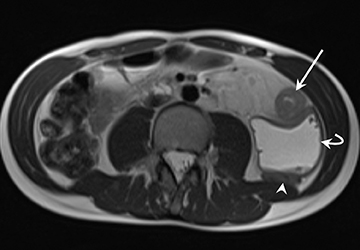
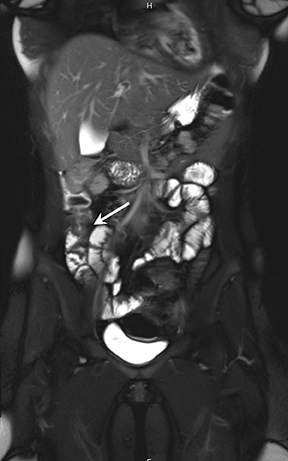
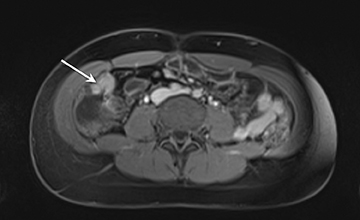
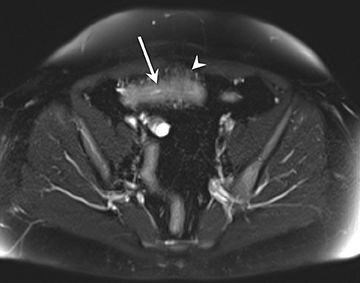
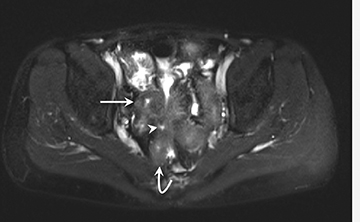
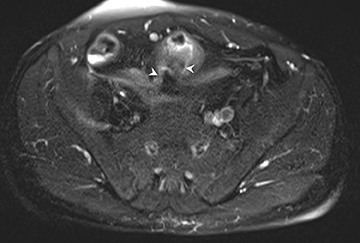
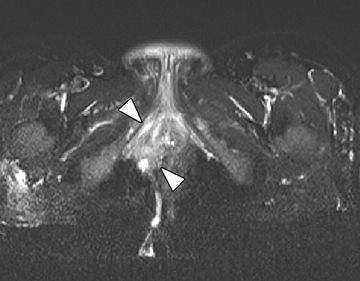
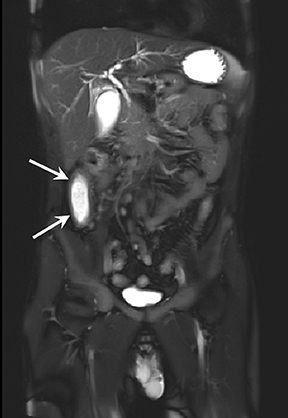
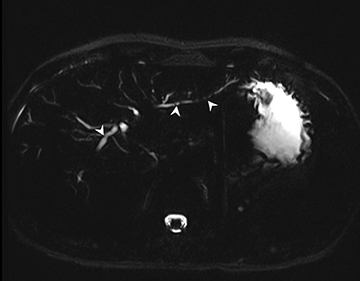
1) Active inflammation: High-signal-intensity edema and inflammatory fluid on T2W-SPAIR fat-suppressed images show enhancement on postgadolinium T1W images coupled with bowel wall thickening (Figure 1).
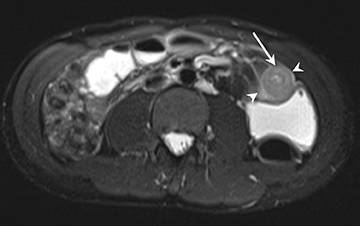
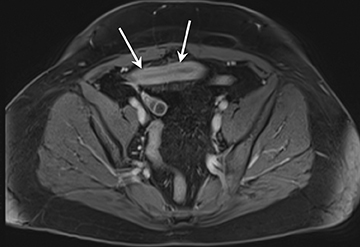
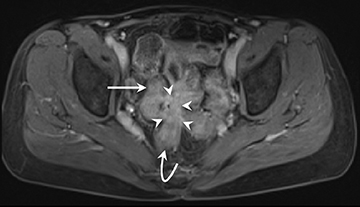
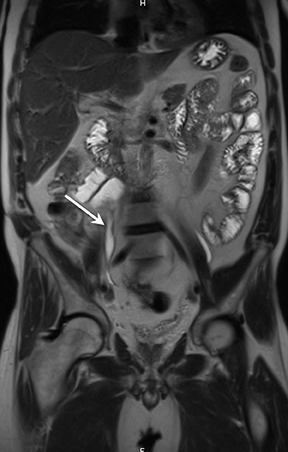
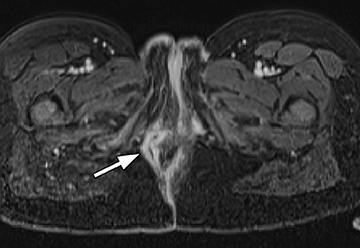
2) Chronic disease without active inflammation: Low-signal-intensity fibrosis and chronic changes on T2W-SPAIR fat-suppressed images with possible stenosis and obstruction. plus bowel-wall thickening and delayed enhancement on postgadolinium T1W images (Figure 2).
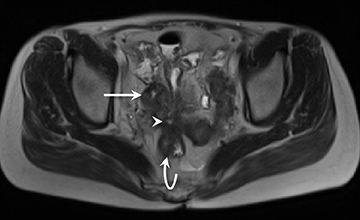
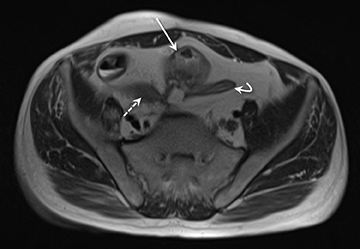
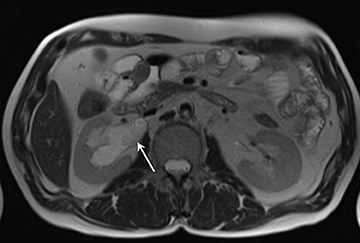
3) Chronic disease with active inflammation (overlapping features of 1 and 2): Active inflammation with thickened bowel wall showing both increased T2 signal and retained contrast on T1W images. Resolution of the elevated T2 signal is a marker of targeted therapeutic change, monitored on longitudinal repeat MRE. Chronic disease is determined by residual thickening and retained contrast (Figure 3).15, 24
Complications of CD: Chronic and relapsing disease can manifest with enteric and extra-enteric complications.
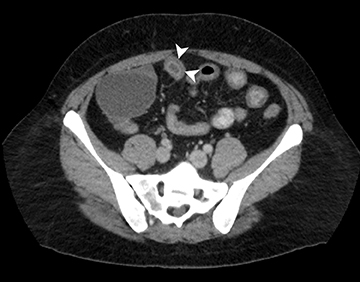
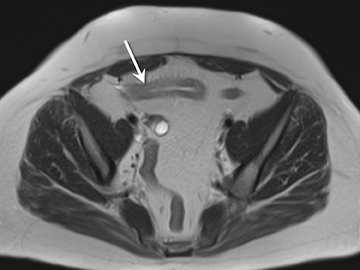
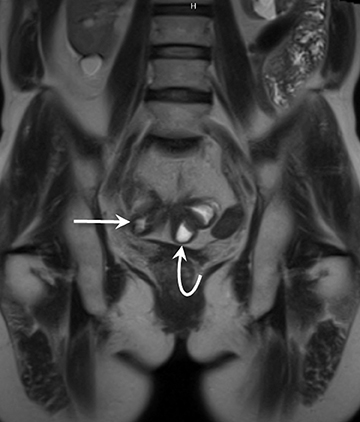
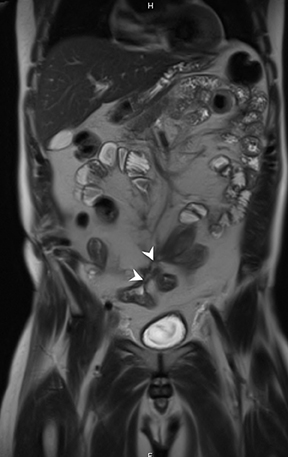
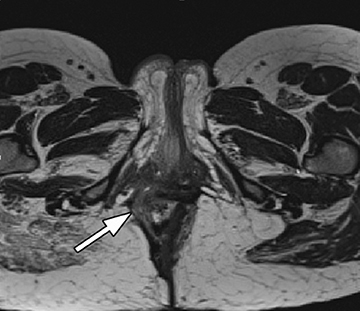
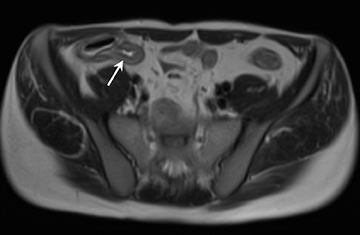
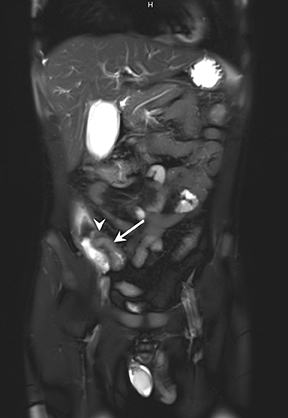
A) Enteric complications: 1) Fistulae, tethering, and strictures (Figures 4 and 5); 2) Bowel obstruction.
B) Extra-enteric complications: 1) Fluid collections, abscesses, and perianal disease (Figure 6); 2) Extra-intestinal manifestations, including, liver or biliary disease such as sclerosing cholangitis (Figure 7), mesenteric vascular thrombi, abdominal masses, tumors, and pancreatic abnormalities.
Active bowel inflammation is promptly depicted on MRE. However, sometimes active inflammation may mask underlying fibrosis related to chronic disease of the bowel wall (Table 2). The presence or absence of underlying fibrosis in this setting is of lesser immediate consequence, as active inflammation requires treatment. In this setting, longitudinal MRE evaluation confirms improvement of active inflammation and evaluates the presence of unmasked chronic fibrotic disease, particularly for potential surgical intervention.
Additional MRE methods and technical considerations
Perfusion characteristics of the bowel wall after intravenous gadolinium administration have been used to estimate disease activity. The combination of T2W images and T1W gadolinium-enhanced images is known to provide high diagnostic accuracy.10, 34 An MRE-based scoring system has been proposed to assess inflammatory activity of the bowel wall, including bowel-wall thickening, lumen narrowing, and the number of peri-intestinal lymph nodes.35 Interpretation based on these parameters is feasible; however, clinical application remains limited by the requirements of image acquisition. Perfusion-type imaging requires specialized scanning techniques or perfectly timed arterial, venous, and delayed-phase, gadolinium-enhanced T1W images (Table 3). This raises the technical challenge of whole abdomen and pelvis imaging without compromising the field of view and overall image quality, as well as free from the effects of respiratory motion or bowel peristalsis. Diffusion weighted imaging (DWI) is loosely related to T2W imaging and experience is developing with DWI techniques that shows potential utility for delineating and improving sensitivity for detecting diseased bowel wall segments and peri-enteric soft tissues.36, 37
Limitations of MRE
An MRE study requires approximately 30 min-45 min. While this duration compares favorably with fluoroscopic studies (SBFT, or enteroclysis), it is approximately twice as long as that needed for CTE. However, considering the entire length of examination, starting with bowel preparation and consumption of pre-scanning oral contrast, the differences in room time become less important than the overall process. Sedation may be required for very young children and patients with claustrophobia. The availability of MRE expertise and access may represent additional relative limitations compared to CTE.
Conclusion
With current optimized T2W and T1W techniques, MRE has evolved to generate reproducible high-quality examinations of the small and large bowel with significantly improved sensitivity and specificity for CD assessment. In the authors’ experience, CT does not match MRE for producing the soft tissue contrast useful to reliably differentiate between inflammation and chronic fibrotic changes. Both processes may look identical on CT. On MRE, T2 signal increases with inflammation and edema, a marker of active CD.23 Single-shot T2 combined with fat-suppression employing the SPAIR technique is critically important to optimizing sensitivity and specificity for active CD on MRE.15,23,24 Earlier studies either did not use fat-suppressed T2 or did not use optimized fat-suppression and, therefore, may not have appreciated the full utility of MRE.16,38,39 Other forms of fat-suppression, using simple inversion-recovery or chemical shift spoiling, will be adversely affected by higher noise, less uniform fat-suppression, or increased through-plane motion sensitivity to bowel peristalsis.24 T2W imaging with fat saturation has shown high accuracy for measuring inflammation and acute disease activity compared to endoscopy, biopsy, and CT. In addition, MRE effectively evaluates severity and extent of submucosal pathology and extra-intestinal complications. MRE is relatively insensitive to early disease changes that are restricted to the mucosa,40 MRE may be combined with endoscopy, capsule endoscopy, or biopsy. The combination of MRE and endoscopic techniques provides a comprehensive examination of inflammatory bowel pathology, including the evaluation of both mucosal and submucosal disease. Further improvements in MRE may be expected through refinements in fat suppression, which is achievable using multi-echo Dixon 3D GRE, or DWI techniques, for quantitative assessment of edema and inflammation, markers of disease activity. We predict eventual integration of MRE into routine CD activity scoring for longitudinal monitoring and management of therapeutic interventions.
References
- Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284.
- Van Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol. 2003;98:332–339.
- Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National cooperative Crohn’s Disease study. Gastroenterology. 1976;70:439–444.
- Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514.
- Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991; 12:439–447.
- Schunk K, Kern A, Oberholzer K, et al. Hydro-MRI in Crohn’s disease: Appraisal of disease activity. Invest Radiol. 2000;35:431–437.
- Miao YM, Koh DM, Amin Z, et al. Ultrasound and magnetic resonance imaging assessment of active bowel segments in Crohn’s disease. Clin Radiol. 2002;57:913–918.
- Koh DM, Miao Y, Chinn RJ, et al. MR imaging evaluation of the activity of Crohn’s disease. AJR Am J Roentgenol. 2001;177:1325–1332.
- Martinez MJ, Ripolles T, Paredes JM, et al. Assessment of the extension and the inflammatory activity in Crohn’s disease: Comparison of ultrasound and MRI. Abdom Imaging. 2009;34:141–148.
- Florie J, Wasser MN, Arts-Cieslik K, et al. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol. 2006;186:1384–1392.
- Del Vescovo R, Sansoni I, Caviglia R, et al. Dynamic contrast enhanced magnetic resonance imaging of the terminal ileum: differentiation of activity of Crohn’s disease. Abdom Imaging. 2008;33:417-424.
- Madsen SM, Thomsen HS, Munkholm P, et al. Inflammatory bowel disease evaluated by low-field magnetic resonance imaging. Comparison with endoscopy, 99mTc-HMPAO leucocyte scintigraphy, conventional radiography and surgery. Scand J Gastroenterol. 2002;37:307-316.
- Laghi A, Borrelli O, Paolantonio P, et al. Contrast enhanced magnetic resonance imaging of the terminal ileum in children with Crohn’s disease. Gut. 2003; 52:393–397.
- Alexopoulou E, Roma E, Loggitsi D, et al. Magnetic resonance imaging of the small bowel in children with idiopathic inflammatory bowel disease: Evaluation of disease activity. Pediatr Radiol. 2009;39:791–797.
- Martin DR, Lauenstein T, Sitaraman SV. Utility of magnetic resonance imaging in small bowel Crohn’s disease. Gastroenterology. 2007; 133:385–390.
- Albert JG, Martiny F, Krummenerl A, et al. Diagnosis of small bowel Crohn’s disease: A prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005; 54:1721–1727.
- Golder SK, Schreyer AG, Endlicher E, et al. Comparison of capsule endoscopy and magnetic resonance (MR) enteroclysis in suspected small bowel disease. Int J Colorectal Dis. 2006;21:97–104.
- Tillack C, Seiderer J, Brand S, et al. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1219–1228.
- Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: Comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–761.
- . Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113-121.
- Schmidt S, Lepori D, Meuwly JY, et al. Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: Interobserver agreement and sensitivity by means of ‘sign-by-sign’ correlation. Eur Radiol. 2003;13:1303-1311.
- Low RN, Francis IR, Politoske D, Bennett M. Crohn’s disease evaluation: Comparison of contrast-enhanced MR imaging and single-phase helical CT scanning. J Magn Reson Imaging. 2000;11:127-135.
- Udayasankar UK, Martin D, Lauenstein T, et al. Role of spectral presaturation attenuated inversion-recovery fat-suppressed T2-weighted MR imaging in active inflammatory bowel disease. J Magn Reson Imaging. 2008;28:1133-1140.
- Lauenstein TC, Sharma P, Hughes T, et al. Evaluation of optimized inversion-recovery fat-suppression techniques for T2-weighted abdominal MR imaging. J Magn Reson Imaging. 2008;27:1448-1454.
- Jaffe TA, Gaca AM, Delaney S, et al. Radiation doses from small-bowel follow-through and abdominopelvic MDCT in Crohn’s disease. AJR Am J Roentgenol. 2007;189:1015–1022.
- Martin DR, Danrad R, Herrmann K, et al. Magnetic resonance imaging of the gastrointestinal tract. Top Magn Reson Imaging. 2005;16:77-98.
- Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn disease: Location-matched histologic validation of MR imaging features. Radiology. 2009;252:712-720.
- Fornasa F, Benassuti C, Benazzato L. Role of magnetic resonance enterography in differentiating between fibrotic and active inflammatory small bowel stenosis in patients with Crohn’s disease. J Clin Imaging Sci. 2011;1:35.
- Ha CY, Kumar N, Raptis CA, et al. Magnetic resonance enterography: Safe and effective imaging for structuring Crohn’s disease. Dig Dis Sci. 2011; 56:2906–2913.
- Negaard A, Paulsen V, Sandvik L, et al. A prospective randomized comparison between two MRI studies of the small bowel in Crohn’s disease, the oral contrast method and MR enteroclysis. Eur Radiol. 2007;17:2294–2301.
- Ajaj W, Lauenstein TC, Langhorst J, et al. Small bowel hydro-MR imaging for optimized ileocecal distension in Crohn’s disease: Should an additional rectal enema filling be performed? J Magn Reson Imaging. 2005; 22:92-100.
- Narin B, Ajaj W, Gohde S, et al. Combined small and large bowel MR imaging in patients with Crohn’s disease: A feasibility study. Eur Radiol. 2004;14:1535-1542.
- Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Prassopoulos P. MR enteroclysis: Technical considerations and clinical applications. Eur Radiol. 2002; 12:2651-2658.
- Maccioni F, Bruni A, Viscido A, et al. MR imaging in patients with Crohn disease: Value of T2- versus T1-weighted gadolinium- enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology. 2006;238:517–530.
- Ajaj WM, Lauenstein TC, Pelster G, et al. Magnetic resonance colonography for the detection of inflammatory diseases of the large bowel: Quantifying the inflammatory activity. Gut. 2005;54:257–263.
- Oto A, Kayhan A, Williams JT, et al. Active Crohn’s disease in the small bowel: Evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33:615-624.
- . Neubauer H, Pabst T, Dick A, et al. Small-bowel MRI in children and young adults with Crohn disease: Retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol. 2012. [Epub ahead of print].
- Shoenut JP, Semelka RC, Magro CM, et al. Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. J Clin Gastroenterol. 1994;19:31–35.
- Sempere GA, Martinez Sanjuan V, Medina Chulia E, et al. MRI evaluation of inflammatory activity in Crohn’s disease. AJR Am J Roentgenol. 2005; 184:1829–1835.
- Lawrance IC, Welman CJ, Shipman P, Murray K. Small bowel MRI enteroclysis or follow through: Which is optimal? World J Gastroenterol. 2009;15:5300–5306.
Citation
The diagnostic role of magnetic resonance enterography in Crohn’s disease: An updated review of techniques, interpretation, and . Appl Radiol.
December 4, 2013