The vomiting infant: Surgical causes of emesis
Images






Vomiting in the infant is a common and nonspecific symptom. Most causes of vomiting in this age group are benign and self-limited, including gastroesophageal reflux, milk allergies, and gastroenteritis. However, several causes constitute medical emergencies and require surgical intervention. In this article, we will explore 4 surgical causes of vomiting in the infant, giving attention to clinical presentation, imaging evaluation, and management, where applicable. The goal is the creation of a work-up algorithm through an understanding of the pathologies, the patterns, and the pitfalls.
Hypertrophic pyloric stenosis
Hypertrophic pyloric stenosis (HPS) is a form of gastric outlet obstruction related to overgrowth of the circular muscle of the pyloric channel. It typically presents in infants in the first 3 to 6 weeks of life as nonbilious vomiting, which has become projectile in character, leading to dehydration as the obstruction becomes complete. The incidence is higher in firstborn males, and those with a positive family history are predisposed.
The diagnosis may be evident based on the patient's medical history and physical examination alone. If an olive-sized mass is palpable in the midepigastrium, the surgeon often will not request a radiographic study.1 That notwithstanding, since gastroesophageal reflux can masquerade as HPS, most clinicians want imaging confirmation.
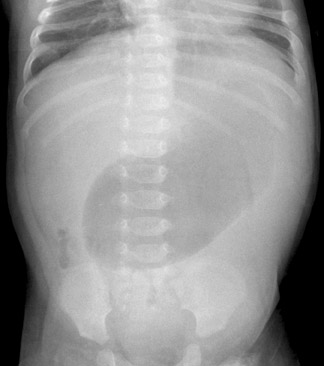
All imaging evaluations for HPS should begin with a radiograph of the abdomen. If the stomach is distended and fluid-filled, even after 2 hours of fasting, the diagnosis should be suspected, particularly if little gas is seen in the bowel (Figure 1). A normal radiograph does not exclude HPS, however.
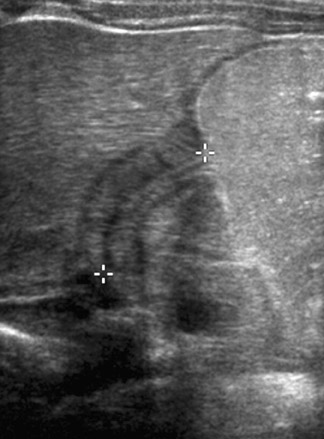
Further evaluation can be accomplished with either an ultrasound or an upper gastrointestinal series (UGI). In our practice, we advocate ultrasound as the initial imaging approach, since it allows direct visualization of the pyloric channel and measurement of channel length and muscle thickness.1 Studies vary as to appropriate upper normal measurements; we denote as abnormal a muscle thickness >3.2 mm and a channel length >16 mm, as these limits provide an acceptable positive predictive value without a significant number of false-positive examinations (Figure2-5 Pitfalls do exist in obtaining the necessary images for diagnosis. The gastric antrum can be mistaken for the pylorus if the stomach is not distended. This issue can be overcome by allowing the infant to ingest Pedialyte (Ross Products, Columbus, OH) during the examination. Not only does the Pedialyte distend the stomach, it also provides a sonographic window that makes the anatomic landmarks more apparent. The improved window also minimizes the second pitfall, which is overestimating muscle thickness by scanning in an oblique plane. Finally, pylorospasm can cause temporary thickening of the pyloric muscle; therefore, real-time observation should be of adequate duration.6 If measurements change and if fluid is observed passing through the channel, the examination is normal.1,3,7
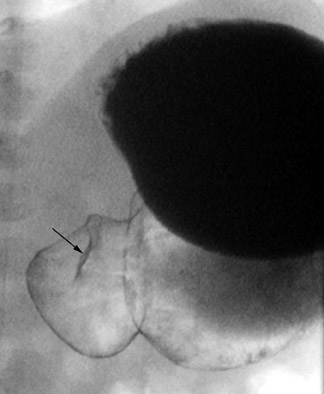
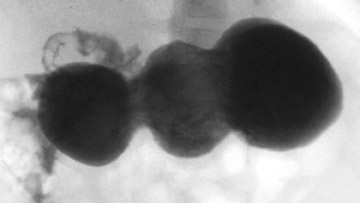
Diagnosis of HPS can be accomplished by a UGI as well. While it provides only an indirect assessment of the pyloric channel, it has the added benefits of offering fuller anatomic evaluation of the stomach and duodenum and functional assessment for gastroesophageal reflux. We recommend passing a nasogastric tube for this examination-for aspirating gastric contents, which can be substantial, and for better control of the contrast medium. Approximately 5 to 10 mL of barium are injected via the enteric tube, followed by 60 to 120 mL of air. A small amount of contrast passing through a narrowed, elongated pyloric channel constitutes the string sign, the most specific and helpful radiographic finding by this technique (Figure 3). The shoulder sign, the impression of the hypertrophied pyloric muscle on the distended gastric antrum, is also helpful and is more readily generated (Figure 4). Other suggestive findings include the caterpillar sign of the hyperperistaltic stomach (Figure 5) and the olive pit sign, which is formed from the pyloric muscle impression upon the antrum seen en face with a tiny amount of contrast at the orifice. As with ultrasound imaging, pylorospasm can be a pitfall; findings must remain constant to diagnose HPS.8,9
Malrotation with midgut volvulus
Malrotation is an error of embryologic development. In the normal course of events, the bowel of the developing embryo herniates into the extraembryonic coelomic cavity, where it makes a 90˚ cephalocaudal rotation before returning into the intra-abdominal cavity and completing a 270˚ counterclockwise rotation. The resulting relationship of small bowel and colon creates a wide mesenteric root, fixed in the left upper quadrant and in the right lower quadrant. Deviation from this developmental pathway may result in a narrow mesenteric attachment, predisposing the patient to midgut volvulus and bowel ischemia or necrosis.10
Bilious vomiting is the hallmark of malrotation with midgut volvulus, either intermittent or fixed.11,12 Although any obstruction distal to the sphincter of Oddi can lead to bilious vomiting, malrotation is the diagnosis to exclude because of the issue of vascular compromise to the bowel. The question of malrotation requires an emergent answer, and a UGI is the examination of choice. A normal abdominal radiograph does not exclude the diagnosis.9,13-15
The UGI is performed via an enteric tube with the tip positioned in the antropyloric region. Five to 10 mL of barium contrast medium are injected, followed by adequate air to push the contrast through the pylorus and duodenum. This technique limits the amount of contrast used in an infant who may require an emergent laparotomy. Fluoroscopic spot images are obtained in the right anterior oblique, anteroposterior, and left lateral projections to show the duodenal C-loop, duodenojejunal junction (DJJ), and retroperitoneal course of the duodenum, respectively. The duodenum should pass posterior to the stomach with the DJJ lateral to the left pedicles at the level of the duodenal bulb. The DJJ can be as much as 1 vertebral body height inferior to the duodenal bulb and still be considered normal.
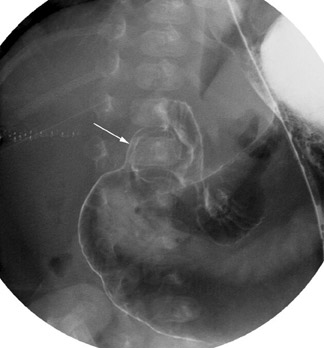
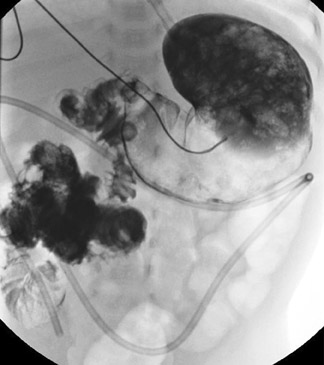
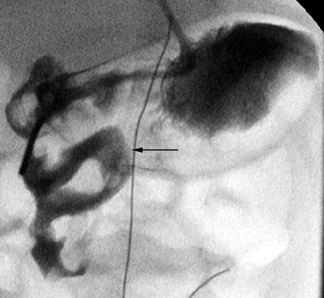
In the case of malrotation with midgut volvulus, the small bowel twists around the axis of the superior mesenteric artery and vein because of its abnormal fixation. The duodenum will not cross the midline, but instead it corkscrews in the right aspect of the abdomen (Figure 6). Classically, the contrast column will terminate in a beaked configuration if the obstruction is complete (Figure 7). Alternative patterns of malrotation exist if the volvulus is intermittent or if the obstruction is related to peritoneal bands (so-called Ladd's bands) crossing anterior to the bowel because of a malpositioned cecum. Ladd's bands create extrinsic compression upon the duodenum.10,14,16
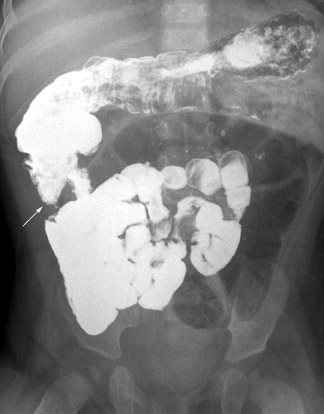
Sometimes the findings on the UGI are equivocal. For example, the apparent DJJ may lie to the left of the midline but lower than 1 vertebral body height; the DJJ may be in the midline (Figure 8A); or contrast may fill jejunal loops that are not clearly to the left of the midline. If a normal interpretation of the examination is in doubt, the contrast should be followed to the cecum with a series of abdominal radiographs obtained over time (Figure 8B). A normally positioned cecum in the face of borderline findings on the fluoroscopic examination does not usually warrant the risks of surgery, as the mesenteric attachment remains adequate.5,13,15,17
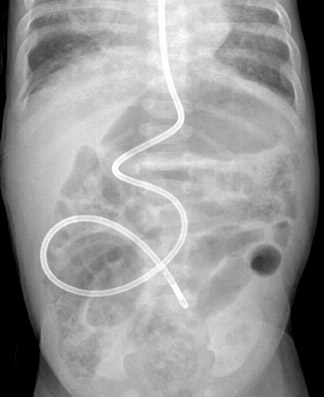
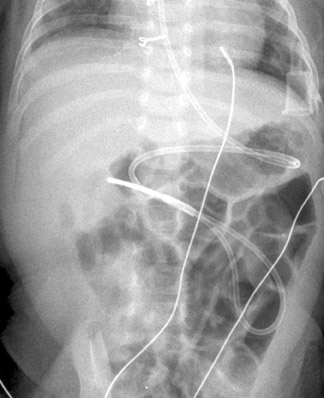
Two other particular situations in the evaluation of suspected malrotation deserve focused consideration, and both stem from the interpretation of the initial abdominal radiograph. First is the abdomen radiograph that shows diffusely dilated bowel loops. This pattern can be seen with complete midgut volvulus and bowel ischemia, but it can also be seen with low intestinal obstructions of other etiologies, including volvulus around an omphalomesenteric duct remnant. With such radiographic findings, the initial fluoroscopic study should be an enema using isosmolar water-soluble contrast medium to determine the position of the cecum and/or the level of obstruction (Figure 9). If this examination is inconclusive, evaluation of the upper gastrointestinal tract should be performed using barium suspension as the contrast medium. The barium is denser and should be distinguishable from any water-soluble contrast remaining in the colon, allowing assessment of the course of the duodenum and the position of the DJJ. The second situation relates to an abnormal feeding tube course as shown by abdominal radiography. Since the tube itself can distort the course of the duodenum, bowel rotation should be assessed with the tube retracted to the duodenal bulb followed by contrast injection (Figures 10 and 11). This practice should prevent unnecessary surgeries for presumed malrotation.
Intussusception
Intussusception is a bowel obstruction that results when 1 bowel loop telescopes into another, usually beginning at the ileocecal valve. In the vast majority of cases, a lead point is never discovered. However, given the epidemiological correlation of viral infections with intussusception incidence, the theory is that activated Peyer's patches in the terminal ileum function as lead points.
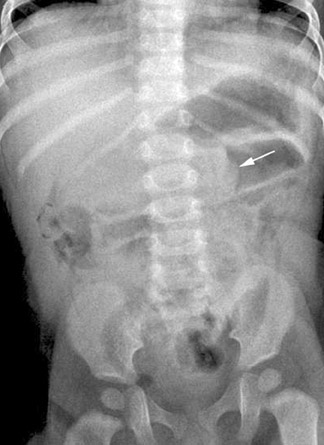
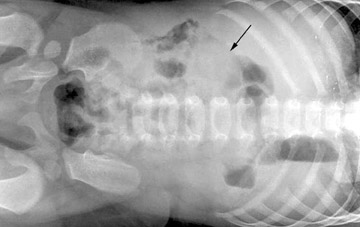
Infants with intussusception typically present with episodes of crampy abdominal pain separated by periods of lethargy. They may vomit frequently, leading to dehydration, and bloody stools are common.11 Imaging evaluation will begin with abdominal radiographs in the anteroposterior and left lateral decubitus projections.18 Air within the cecum effectively excludes the diagnosis. In positive cases, a paucity of gas is seen in the right lower quadrant and sometimes within the entire lower abdomen and pelvis.9 A complete obstruction of at least several hours' duration may yield the classic pattern of dilated bowel loops and stacked air-fluid levels (Figure 12). The identification of a soft tissue mass that silhouettes the inferior liver edge in this clinical setting is pathognomonic (Figure 13).
The infant with presumed intussusception should undergo immediate image-guided enema reduction. The only contraindications to the procedure are severe dehydration, shock, peritonitis, and pneumoperitoneum, any of which make the case a purely surgical one. The enema can be performed with positive oral contrast or with air, with fluoroscopy or ultrasound serving as the imaging modality. Of these choices, we advocate pneumatic reduction under fluoroscopy for its speed, its clarity, and its ease. Additionally, if the intussusceptum cannot be fully reduced, an air-filled colon rather than a barium-filled colon is more desirable at surgery. Moreover, in the rare event of an intraprocedural bowel perforation, the volume of fecal contents may be less with air as the reduction agent than with barium.9,19
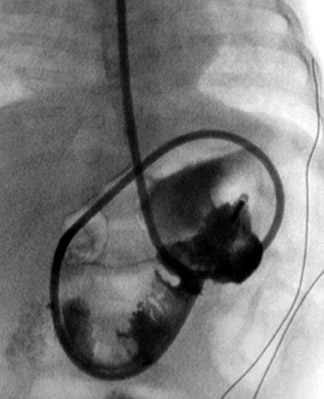
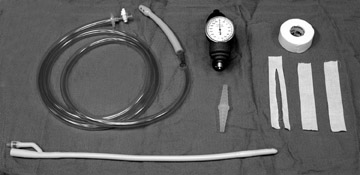
The air reduction technique utilizes a Foley catheter (of the largest caliber that is appropriate for the patient), a pneumatic kit (GRI Medical Products, Inc., Cave Creek, AZ) (which is composed of an adapted sphygmomanometer with pressure gauge and tubing), and Christmas tree adapters (Figure 14). The apparatus is assembled, and the catheter is taped securely in the patient's rectum. Air is then insufflated into the colon, where it encounters the intussusceptum and, it is hoped, pushes the mass back through the ileocecal valve (Figure 15). Resting pressures should be maintained <120 mm Hg with the patient quiet. Of note, the patient is not sedated for this procedure because the child can offer some resistance to the insufflation, which serves as another check against overinflating the colon. Reduction is complete when air has refluxed into the distal ileum, as evidenced by fluoroscopy. If the mass is no longer movable, even after 2 or 3 more attempts, that situation also serves as the end point of the examination; completion of the reduction will occur in the operating room. Finally, bowel perforation will also terminate the examination and lead to surgery. In the rare event of bowel perforation, an 18-gauge needle can be placed approximately 2 cm below the umbilicus to obviate the risk of pneumoperitoneum.
Fewer than 5% of cases of intussusceptions recur. Most of those recurrences are within the first 24 hours after the initial reduction, supporting the practice of a 24-hour hospital stay after successful therapeutic enema.11 Recurrences, especially more than 1, are suggestive of a lead point. These can include a Meckel's diverticulum, a duplication cyst, a polyp, or a bowel or mesenteric neoplasm, such as Burkitt's lymphoma. In infants, Meckel's diverticulum would be the most common.19
Incarcerated inguinal hernia
Incarcerated inguinal hernia is one of the most common causes of bowel obstruction in infancy. These occur much more commonly in boys than in girls, generally being of the indirect type via a patent processus vaginalis. Premature infants have a greater risk for this event than do term infants.20 As with malrotation and intussusception, bilious vomiting can be the presenting sign.
The role of the radiologist in diagnosing incarcerated inguinal hernia is variable. Patients present with signs of obstruction, such as vomiting and abdom-inal distention, plus an inguinal-scrotal mass or fullness. If the clinician cannot reduce the hernia sac, the diagnosis is made. Sometimes, however, the diagnosis is equivocal, and imaging is indicated.
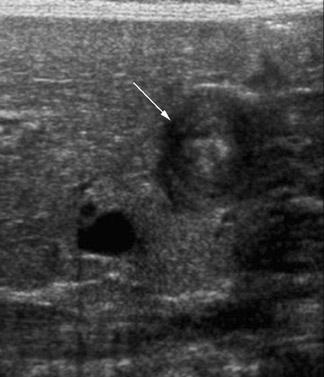
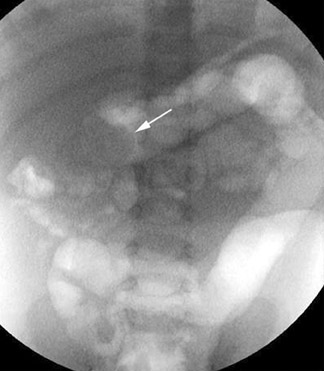
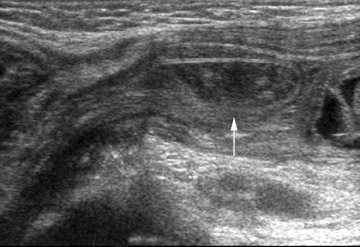
The abdominal radiograph will confirm a mechanical bowel obstruction and a thickened inguinal-scrotal fold (with or without bowel gas within the hemiscrotum) if incarcerated hernia is the etiology of the patient's symptoms. It may also direct the care team to another diagnosis. Ultrasound can provide important additional information through its ability to assess vascular flow to the involved bowel loop within the inguinal ring (Figure 16). Pneumatosis intestinalis can affect incarcerated bowel if the degree of vascular compromise is sufficient, but this finding should be visible on the accompanying radiographs (Figure 17).
Conclusion
The vomiting infant can present a diagnostic challenge. The first question to answer is whether the problem is medical or surgical. The history and physical examination coupled with the abdominal radiograph should direct the next step in management. While the plain radiograph examination cannot fully exclude any of the discussed causes of infantile vomiting, it is crucial for choosing further imaging options, for excluding contraindications to subsequent studies, and for assessing for any unexpected findings. If the initial radiographs suggest an obstruction, but the level is indeterminate, the first fluoroscopic examination should be an enema. If intussusception is in the differential considerations, then the enema should be performed with appropriate technique for an anticipated reduction, whether with air or with positive contrast. If the abdominal radiograph is nonspecific, high obstruction or gastro-esophageal reflux could still explain the symptom profile, making a UGI in order-with the caveat that ultrasound is a more specific test for hypertrophic pyloric stenosis.
Other, rarer causes of vomiting in infants that are related to obstruction include antral and duodenal webs, small bowel stenosis, complicated Meckel's diverticulum, and appendicitis. The former are generally apparent on fluoroscopic examination with careful attention to technique and spot compression. Complicated Meckel's diverticulum and appendicitis may require abdominal ultrasound or computed tomography for diagnosis. Nevertheless, with an orderly approach in obtaining imaging studies, we can diagnose or exclude the common problems readily and move on to other tools in our arsenal to identify the rare conditions.
References
- Hernanz-Schulman M, Sells LL, Ambrosino MM, et al. Hypertrophic pyloric stenosis in the infant without a palpable olive: Accuracy of sonographic diagnosis. Radiology. 1994;193:771-776.
- Argyropoulou MI, Hadjigeorgi CG, Kiortsis DN. Antropyloric canal values from early prematurity to full-term gestational age: An ultrasound study. Pediatr Radiol. 1998;28:933-936.
- Blumhagen JD, Noble HG. Muscle thickness in hypertrophic pyloric stenosis: Sonographic determination. AJR Am J Roentgenol. 1983;140:221-223.
- Bowen A. The vomiting infant: Recent advances and unsettled issues in imaging.Radiol Clin North Am. 1988; 26:377-392. Erratum in:Radiol Clin North Am.1988;26(3):following ix.
- Maclennan AC. Investigation in vomiting children. Semin Pediatr Surg. 2003;12:220-228.
- Cohen HL, Zinn HL, Haller JO, et al. Ultrasonography of pylorospasm: Findings may simulate hypertrophic pyloric stenosis. J Ultrasound Med. 1998;17: 705-711.
- Hernanz-Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227:319-331.
- McCollough M, Sharieff GQ. Abdominal surgical emergencies in infants and young children. Emerg Med Clin North Am. 2003;21:909-935.
- Weinberger E, Winters WD. Abdominal pain & vomiting in infants and children: Imaging evaluation. Compr Ther. 1997;23:679-686.
- Hernanz-Schulman M. Imaging of neonatal gastrointestinal obstruction. Radiol Clin North Am. 1999;37:1163-1186, vi-vii.
- Brennan DF. Bilious vomiting in a 9-month-old infant.Acad Emerg Med.1997;4:706-710.
- Godbole P, Stringer MD. Bilious vomiting in the newborn: How often is it pathologic? J Pediatr Surg. 2002;37:909-911.
- Ford EG, Senac MO Jr, Srikanth MS, Weitzman JJ. Malrotation of the intestine in children. Ann Surg. 1992;215:172-178.
- Kimura K, Loening-Baucke V. Bilious vomiting in the newborn: Rapid diagnosis of intestinal obstruction. Am Fam Physician. 2000;61:2791-2798.
- Powell DM, Othersen HB, Smith CD. Malrotation of the intestines in children: The effect of age on presentation and therapy. J Pediatr Surg. 1989;24: 777-780.
- Sumner TE, Cox TD, Auringer ST. Emergency neonatal gastrointestinal imaging. Appl Radiol.2002; 31(2):9-16.
- Janik JS, Ein SH. Normal intestinal rotation with non-fixation: A cause of chronic abdominal pain. J Pediatr Surg. 1979;14:670-674.
- Smith DS, Bonadio WA, Losek JD, et al. The role of abdominal x-rays in the diagnosis and management of intussusception. Pediatr Emerg Care. 1992;8: 325-327.
- Daneman A, Navarro O. Intussusception. Part 2: An update on the evolution of management. Pediatr Radiol.2004;34:97-108; quiz 187.
- Parker B. Small intestine. In: Kuhn JP, Slovis TL, Haller JO, eds. Caffey’s Pediatric Diagnostic Imaging. Philadelphia, PA: Mosby, 2004:1638-1646.