Ultrasound in pediatric emergencies
Images










Ultrasound (US) imaging has several advantages over other radiologic imaging modalities, particularly in the emergency department (ED). It is a low cost, non-invasive, easily accessible and painless imaging modality that can be quickly performed at the bedside of an unstable or very ill patient. It is easily reproducible and can be repeated multiple times without any risk. However, the greatest advantage of US over other imaging modalities, such as computed tomography (CT), is the absence of ionizing radiation. As evidence of harmful effects of radiation due to CT continues to increase, US is gaining greater acceptance as the imaging modality of choice in the pediatric emergency setting. The main disadvantage of US is operator dependence. This review article highlights the use of US in evaluating common emergency conditions in children presenting to the ED.
Traumatic pathology
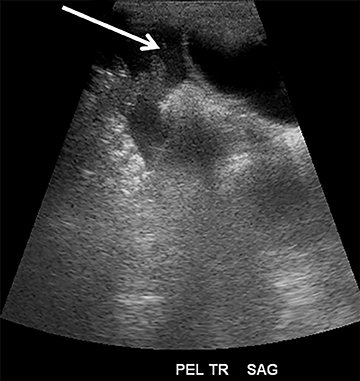
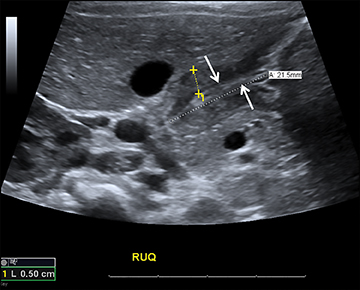
Focused assessment with sonography for trauma (FAST) is a rapidly performed bedside study to identify ectopic abdominal fluid. The four locations typically examined for free fluid include the right upper quadrant (perihepatic space or the Morison’s pouch), the left upper quadrant (perisplenic space), the pericardium and the pelvis. A positive FAST is defined as the presence of fluid, seen as an anechoic volume in dependent regions of the peritoneal cavity (Figure 1). For an unstable patient with blunt trauma, the speed and cost of ultrasound (US) are significantly better than either diagnostic peritoneal lavage or CT. In addition, including US for trauma in the ED results in decreased time to operative care and more efficient utilization of ED resources.1
Abdominal pathology
Malrotation and volvulus
Intestinal malrotation is a developmental abnormality leading to a decrease in the length of the mesenteric root, predisposing the midgut to rotation. One of the most dangerous sequelae of malrotation is volvulus, a medical emergency that causes bowel obstruction, ischemia of and necrosis of the affected intestine. Patients can present with bilious vomiting, failure to thrive, abdominal pain and other nonspecific symptoms. Although an upper gastrointestinal (UGI) contrast study is the gold standard of diagnosis, US can also be useful, particularly when results of the UGI study are equivocal. On US, the anatomical relationship of the superior mesenteric artery (SMA) and superior mesenteric vein (SMV) may be reversed (Figure 2A). Normally, the SMV is to the right of the SMA. With midgut volvulus, the SMV may occupy a position directly anterior or to the left of the SMA. It is important to note, however, that a normal SMA/SMV relationship does not exclude volvulus and the UGI study remains the imaging gold standard. Conversely, some children without volvulus may have a vertical or inverted SMA/SMV relationship.2
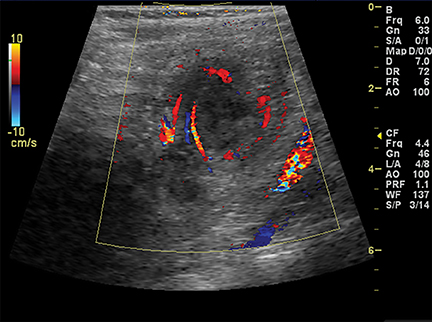
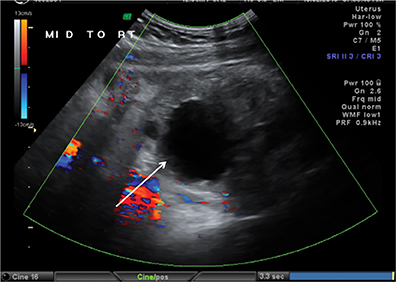
On US, twisting of the mesenteric vessels and superior mesenteric veins (SMV) around the superior mesenteric artery (SMA) can produce a “whirlpool sign” on color Doppler US (Figure 2B).3 Other US signs of volvulus include the “hyperdynamic pulsating SMA” or the “truncated SMA” sign.4,5
Pyloric stenosis
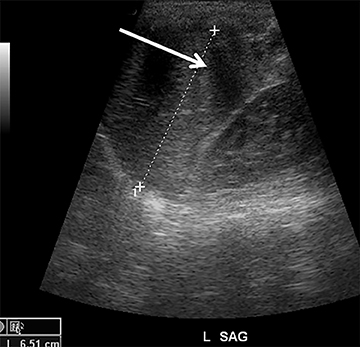
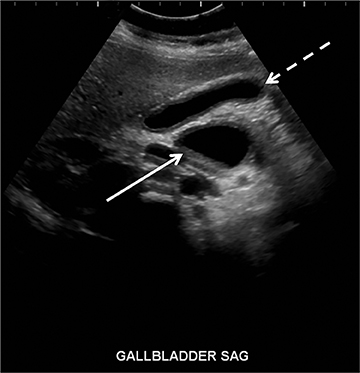
Although not a true emergency, pyloric stenosis is common in the ED. Pyloric stenosis occurs when hypertrophy of the pyloric muscle causes narrowing, resulting in projectile non-bilious vomiting. The physical exam may reveal an olive-shaped mass in the right upper quadrant of the abdomen. US is the modality of choice when suspecting pyloric stenosis.6 The main diagnostic criterion is a measurement of greater than 3mm in the thickness of the muscular layer of a single wall of the pylorus (Figure 3). Elongation of the canal greater than 12mm has also been reported as an abnormal finding, but is less reliable due to the difficulty in achieving reproducible measurements.7 Other findings include hypertrophy of the mucosa, gastric distension with active peristalsis and redundant mucosa protruding through the antrum. Some technical maneuvers which might be useful while performing the US include laying the patient right-side down (allowing gas to move away from the pylorus), use of sugar water to feed (not only helps in calming the baby but also provides a good acoustic medium for the ultrasound beam) and observing the pylorus during the study for any passage of stomach content through the pyloric channel.8
Intussusception
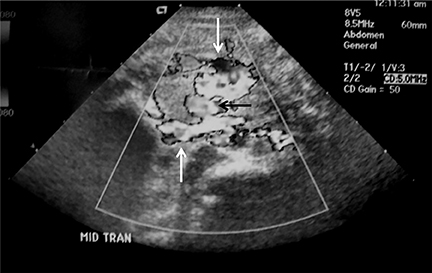
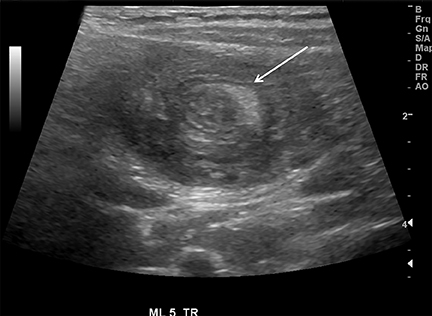
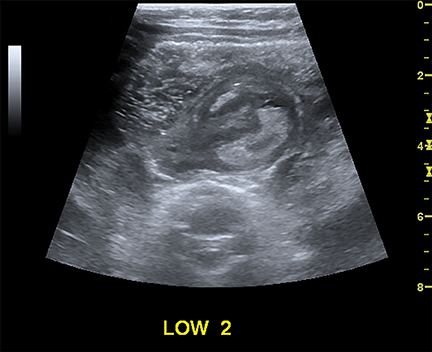
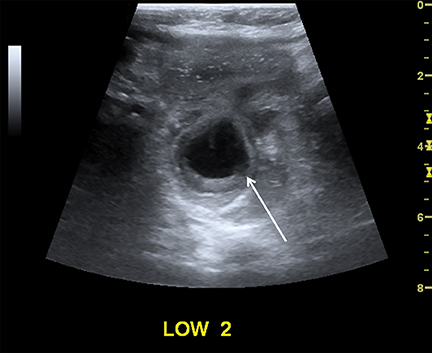
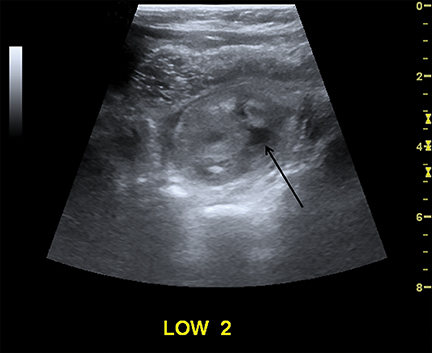
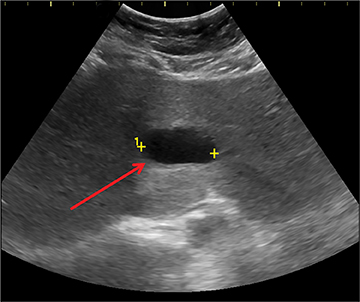
Intussusception occurs when part of the intestine invaginates or telescopes into a distal portion of bowel, causing obstruction. Symptoms include nausea, vomiting, crampy abdominal pain, and rectal bleeding classically described as “red currant jelly.” Most patients are between the ages of 6 months and 2 years. Prognosis is improved by early diagnosis. US has a high sensitivity of 98-100% and specificity of 88-100%.9 US findings include a soft-tissue abdominal mass corresponding to the intussusception. The intussusception appears as a “target” or “donut” sign (Figure 4) created by the receiving bowel loop- intussuscipiens, and the proximal prolapsing bowel loop- intussusceptum, with echogenic intervening mesenteric fat. Another US appearance described is the “pseudokidney” sign, when mesentery containing fat and vessels are dragged into the intussusception, suggesting the US appearance of the renal hilum, with the apparent renal parenchyma formed by the surrounding edematous bowel.10 Ultrasound can also be useful in detecting a lead point, such as lymph nodes, polyps, duplication cysts (Figure 5) and Meckel’s diverticulum, among others. Trapped fluid within the intussusception (Figure 5) and absence of blood flow to the bowel on Doppler imaging have been described as signs of ischemia and decreased reducibility .9 Recently, successful reduction with ultrasound-guided hydrostatic pressure has been performed in some institutions.11
Appendicitis
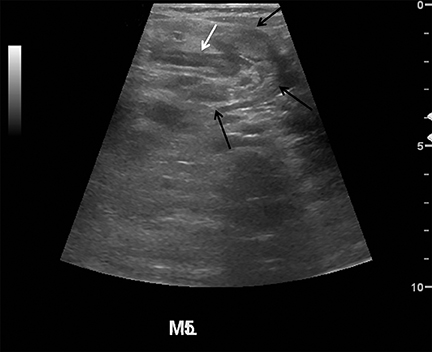
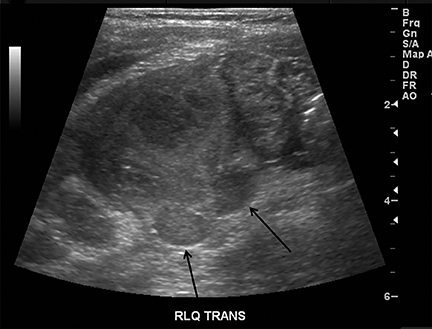
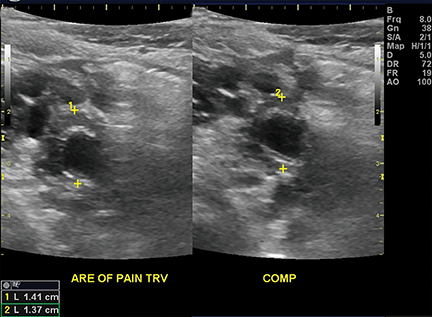
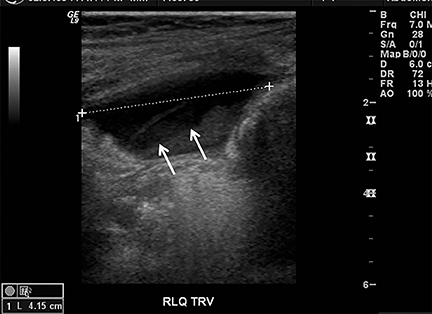
Appendicitis is the most common childhood surgical emergency. The diagnosis is challenging, particularly in younger children, potentially leading to a false negative diagnosis with potential for perforation and other complications. Although primarily a clinical diagnosis, imaging studies can aid in confirmation and reducing the number of negative appendectomies. US has a sensitivity of 88% and specificity of 94% according to a meta-analysis of studies from 1986 to 2004.12 US evaluation is performed using a high frequency linear transducer and graded compression technique. Gentle pressure is applied to the right lower quadrant to displace normal bowel loops and ascertain the position of the nonperistaltic cecum. The appendix usually lies just lateral to the cecum and anterior to the iliac vessels. Positive findings of appendicitis include an outer diameter greater than 6 mm, a noncompressible lumen arising from the base of the cecum, echogenic periappendiceal inflammatory fat changes, an appendicolith or a periappendiceal fluid collection (Figures 6 and 7). Enlarged mesenteric lymph nodes or signs of a perforated appendix such as an abscess can also be seen.
Scrotal pathology
US is the primary imaging modality for evaluating the acute scrotum in children. Common pediatric scrotal diseases seen in the ED include testicular torsion, testicular appendage torsion, epididymitis, orchitis, hernia, hematocele and abscess. Familiarity with ultrasound technique, characteristics and common pitfalls is essential to differentiate these conditions, establish an accurate diagnosis, and initiate treatment.
Testicular torsion
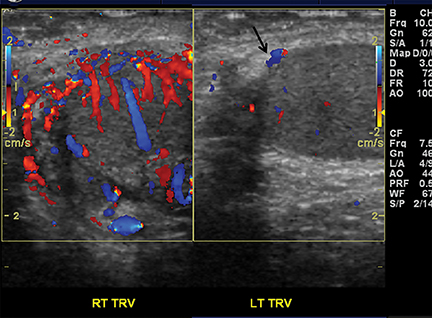
The principle objective in imaging the child with acute scrotal pain is to promptly detect testicular torsion. Testicular torsion results when an abnormally mobile testis twists on the spermatic cord, obstructing its blood supply. Testicular torsion is a surgical emergency since ischemia can lead to testicular necrosis and nonviability if not corrected within 6 hours of onset of symptoms.13Severe testicular pain is the most common presenting symptom.
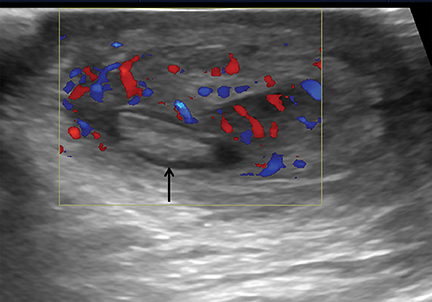
On color Doppler, complete absence of intratesticular blood flow and normal extratesticular blood flow is diagnostic (Figure 8).13,14 The presence of flow within the testis does not exclude torsion, since incomplete vascular obstruction can sometimes occur from intermittent torsion. Using a linear-array high-frequency (7-14 MHz) transducer, the asymptomatic testis is scanned to optimize settings for low flow, resistance and velocity. Once a satisfactory image of the asymptomatic side is obtained, the painful symptomatic side is studied without changing machine settings.15
Gray-scale sonography has no role in diagnosing torsion, but can effectively display the sequelae of testicular ischemia and predict viability.13 During the first 6 hours after symptom onset, when the testis is salvageable, normal architecture and echogenicity is typically preserved. Over the next 24 hours, the testis becomes enlarged, susually hypoechoic or inhomogeneous, associated with edema of the epididymis and scrotal wall. These alterations indicate decreased likelihood of viability. Imaging both testes simultaneously in transverse orientation is the optimal technique to identify subtle sonographic differences (Figure 8).16
Testicular torsion can be a diagnostic challenge because blood flow is often difficult to detect in normal small prepubertal testes. Torsion may be partial or intermittent so arterial flow is not necessarily absent. The testis can also undergo spontaneous detorsion and ischemia may be secondary to other conditions (vasculitis, trauma, epididymitis-ochitis).13,16 Hydrocele and scrotal skin thickening are common findings in the majority of these conditions and thus nonspecific.
Torsion of testicular appendage
In a child with an acute scrotum, torsion of a testicular appendage represents the most common cause of scrotal pain.17Testicular appendages are remnants of the paramesonephric duct and are usually located at the superior testicular or epididymal head.18 Patients are typically under 13 yrs. of age and the onset of pain is more gradual than that seen with testicular torsion; thus, patients often present days after symptoms develop. An avascular structure in an area of increased vascularity, separate from the typically enlarged testis and epididymis is the characteristic appearance of testicular appendage torsion (Figure 9).17 Torsion can lead to increased vascularity in the epididymis and testis and thus may mimic epididymitis and/or orchitis. The torsed appendage becomes more echogenic with time and can eventually calcify or slough off as a calcified loose body between the layers of the tunica vaginalis; referred to as a “scrotal pearl.”
Epididymitis
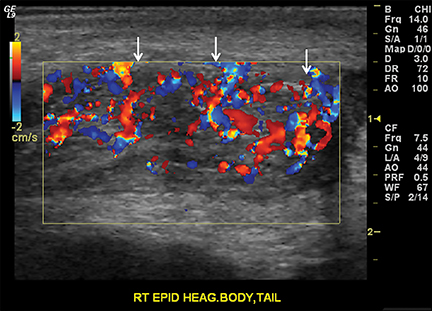
Epididymitis is more common in young adults secondary to sexually transmitted pathogens. However, it also occurs in children and is usually idiopathic, due to retrograde urinary tract infection or may be related to an underlying urogenital anomaly.18 In the acute setting, the epididymis appears enlarged and heterogeneous on gray-scale US and demonstrates increased flow on color Doppler (Figure 10).14,16 The adjacent testis is concurrently involved in 20% of cases, occurring more commonly in adults.14,18 A heterogeneous, enlarged and hyperemic testis is characteristic of orchitis. Orchitis without epididymitis is typical of infection by the paramyxovirus, which causes mumps.15 Complications include abscess and infarction, both of which can be easily mistaken for torsion.19
Testicular trauma
In the setting of trauma, accurately diagnosing testicular rupture is important, since treatment involves urgent surgical repair. On gray-scale US, the testicle appears heterogeneous with irregular contours and the tunica albuginea is disrupted (Figure 11A, B).18,20 Hematocele is the most common finding following trauma, which initially appears echogenic becoming hypoechoic as it evolves. The hematocele is avascular on color Doppler.19
Hernia
Intrascrotal inguinal hernia occurs through the patent processus vaginalis. Imaging should begin with the patient supine and then standing. The bowel or omentum is visualized separately from the normal testis and epididymis. Absence of peristalsis on real-time scanning is worrisome for incarceration, another cause of acute scrotal pain.
Pelvic pathology
Ovarian
US is the imaging modality of choice for evaluating the pediatric female pelvis to exclude or diagnose emergency surgical conditions, including ovarian torsion, ectopic pregnancy and abscess. Functional ovarian (follicular) cysts result from failure of involution during the normal menstrual cycle. Bleeding into or rupture of a functional ovarian cyst is a cause of pain and may mimic acute appendicitis.21 Non-hemorrhagic ovarian cysts appear as avascular, anechoic, thin-walled masses with posterior acoustic enhancement. Most cysts are small and resolve without treatment, but some may be large (up to 6 cm), in which case follow-up is recommended to ensure resolution. The US appearance of hemorrhagic cysts depends on the age of the blood, but typically they appear as avascular complex adnexal masses with septations and internal echoes in a “lace-like pattern” with some degree of through transmission.22,23
Congenital anomalies
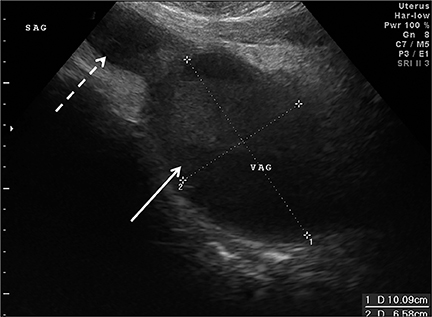
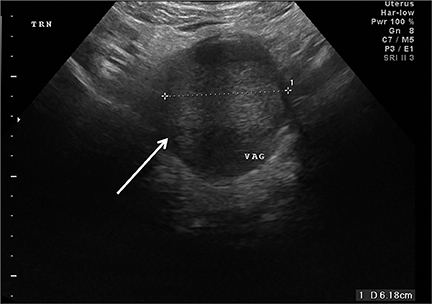
Congenital anomalies are not infrequently encountered in the ED as a cause of pain in the female child or adolescent. Hydrocolpos, hydrometrocolpos and hematametrocolpos in adolescents are typically caused by an imperforate hymen, leading to filling of the uterus and/or vagina with fluid and blood. US demonstrates a pelvic cystic mass with a fluid-debris level (Figure 12A-C).21,22 Many cases of hydrometrocolpos in the neonate are associated with a urogenital sinus or cloacal malformation.21, 23 Failure of the Mullerian or para-mesonephric ducts to reach the urogenital sinus causes accumulation of uterine secretions proximal to the vaginal occlusion. This anomaly may present as a bulging mass between the labia in neonates or with primary amenorrhea and cyclical lower abdominal pain in adolescents.22
Ovarian torsion
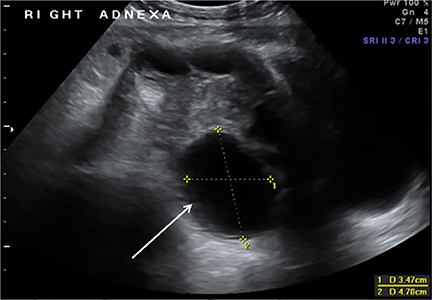
Ovarian torsion is more common in patients with predisposing lesions, such as ovarian cysts or masses (teratomas), and is due to excessive mobility of the ovary.22,24 Presentation is often confusing clinically and includes abrupt onset of severe lower abdominal pain usually preceded by intermittent pain or palpation of a pelvic mass.23 Torsion initially occludes the venous circulation and, if untreated, progresses to occlude the arterial circulation. On US, the ovary appears diffusely enlarged with multiple peripheral follicles. (Figure13A-B).25 Additional findings include a complex pelvic mass, free fluid in the pouch of Douglas and absence of flow on color Doppler. However, lack of flow on color Doppler is not a reliable diagnostic criterion, as arterial flow has been seen in surgically proven ovarian torsion likely due to a dual arterial supply or preceding venous thrombosis.21,24,25
Pancreaticobiliary pathology
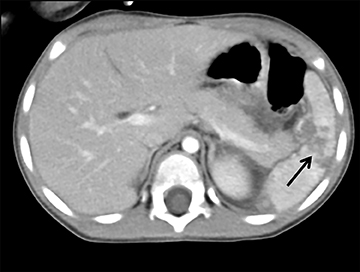
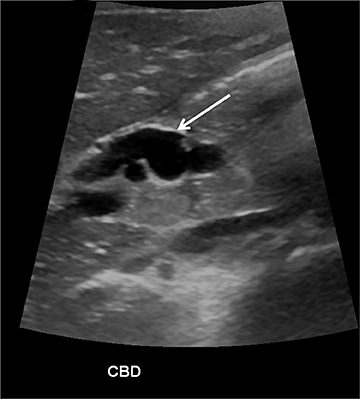
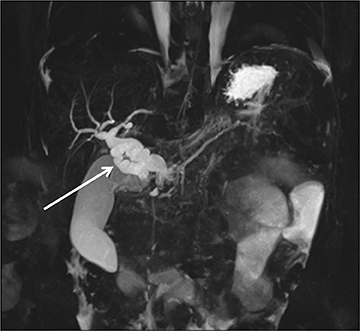
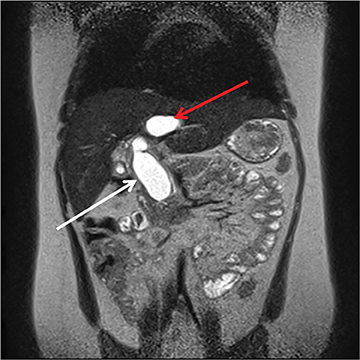
The pancreas and biliary system are well evaluated with US in the acute setting. Imaging findings in pancreatitis are similar to adults and include diffuse or focal, often hypoechoic, pancreatic enlargement and dilatation of the pancreatic duct.26-28 Pseudocyst, pancreatic abscess, necrosis and hemorrhage are the main complications. However, the causes differ in the pediatric setting and are usually idiopathic or related to trauma or congenital structural anomalies such as pancreatic divisum and choledochal cysts. Choledochal cysts are congenital cystic malformations of the biliary tree. Patients typically present with episodic pain, jaundice, a right upper quadrant mass or clinical pancreatitis.26 US can easily localize and measure the degree of biliary dilatation and differentiate among the five types of biliary congenital choledochal cysts (Figures 14 and 15).
Renal pathology
Pediatric renal disease, including pyelonephritis, ureteropelvic junction (UPJ) obstruction and rarely nephrolithiasis, is a frequent cause of abdominal pain. Although the sensitivity of US in detecting acute pyelonephritis is low, the sensitivity for complications, including abscess and pyonephrosis, is quite high.29 On US, the infected kidney appears enlarged with focal or diffuse areas of abnormal echogenicity and loss of corticomedullary differentiation. Power Doppler increases sensitivity by detecting decreased perfusion in the affected area of the kidney.30,31
UPJ obstruction can be secondary to intrinsic causes or extrinsic compression secondary to bands, kinks or aberrant vessels. Patients may present with a palpable mass, urinary tract infection or hematuria. US can detect a dilated renal pelvis communicating with dilated calyces, however, the renal pelvis is dilated out of proportion to the calyces and the distal ureter is not seen. It is essential to differentiate UPJ obstruction from a multicystic dysplastic kidney, which very often occurs in the contralateral kidney and appears as multiple non-communicating cysts of varying sizes.31-33
Cranial pathology
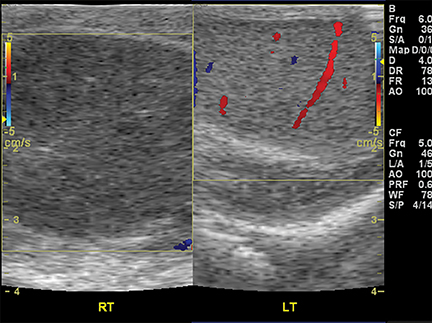
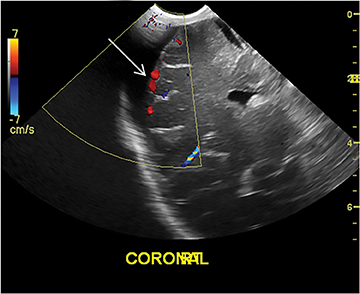
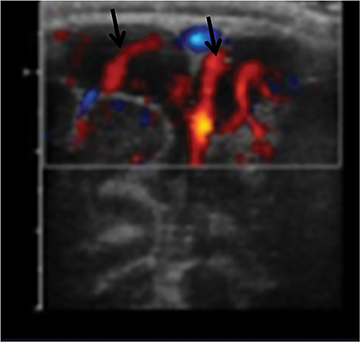
In a young infant (with an open anterior fontanelle), presenting to the ER with an enlarging head circumference, cranial US can be used not only to rule out a parenchymal abnormality (hemorrhage, hydrocephalus, tumor, etc.) but also to differentiate benign enlargement of subarachnoid spaces (BESS) from subdural collections, particularly hematomas. BESS refers to excessive CSF accumulation in the subarachnoid spaces (particularly in the frontal regions) in infants.34 The exact cause is unknown, but arachnoid villi immaturity has been hypothesized as one of the probable causes.35 Identifying subdural hematomas as the cause of extra-axial collections is particularly important in the setting of non-accidental trauma. Doppler imaging is extremely useful in differentiating BESS from subdural hematoma (Figure 16).36 In BESS, multiple vessels can be seen traversing the extra-axial collections, which helps in localizing collections to the subarachnoid space. Collections in the subdural space, on the other hand, displace vessels towards the surface of the brain.
Musculoskeletal pathology
US applications in MSK pathology include evaluation of joint effusions (particularly of the hip), guidance for percutaneous drainage and localization of non-radiopaque foreign bodies.
Hip effusion
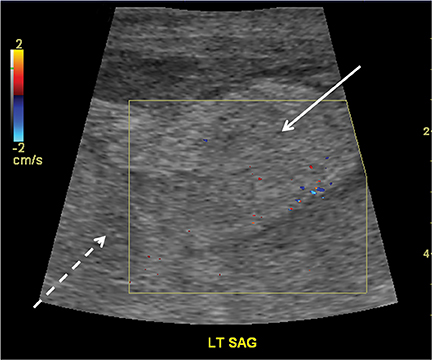
Hip effusions have various etiologies. However, when a pediatric patient presents with fever, pain, and refusal to bear weight on one limb, septic arthritis or transient synovitis are the most likely diagnoses. Transient or toxic synovitis is a self-limiting inflammation of the joint space precipitated by a prior infection (classically an upper respiratory infection) or allergy.36 A hip effusion may or may not always be present in transient synovitis (Figure 17). In septic arthritis, it is extremely important to diagnose and treat quickly due to the potential devastating consequence of joint destruction.37 Usually, joint effusions are clear in transient synovitis and contain debris (due to purulent fluid) in cases of septic arthritis.38 However, this may not always be the case and the currently accepted mode of practice is US- guided aspiration of fluid, even if clear, to exclude the possibility of septic arthritis.39,40 If no fluid is seen on the US, the cause is presumed to be due to transient synovitis, particularly if other clinical signs and symptoms favor the diagnosis and no invasive procedure is necessary.
Foreign bodies
A high-frequency linear transducer is used for the localization of foreign bodies.41 Usually, a “stand-off pad” is used for better sound transmission and an improved view of the underlying soft tissues. Foreign bodies usually appear hyperechoic in relation to the surrounding soft tissues.41 Material such as wood or plastic tends to produce shadowing, whereas metallic objects produce reverberation or a “comet-tail” artifact.42,43 Color Doppler can be utilized to ensure that there are no vascular structures adjacent to the foreign body.43
Conclusion
Ultrasonography has many applications in the evaluation of pediatric patients presenting to the ED with both traumatic and non-traumatic emergencies. Because of its rapidity, ease of use and absence of ionizing radiation, US has not only been used to make or exclude diagnoses, but it has also become the modality of choice in the imaging of both the stable and unstable pediatric patient in the ED.
References
- Melniker LA, Leibner E, McKenney MG, et al. Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: The first sonography outcomes assessment program trial. Ann Emerg Med. 2006;48:227-235.
- Zerin JM, DiPietro MA. Superior mesenteric vascular anatomy at US in patients with surgically proved malrotation of the midgut. Radiology. 1992;183:693-694.
- Shimanuki Y, Aihara T, Takano H, et al. Clockwise whirlpool sign at color Doppler US: An objective and definite sign of midgut volvulus. Radiology. 1996;199:261-264.
- Smet MH, Marchal G, Ceulemans R, Eggermont E. The solitary hyperdynamic pulsating superior mesenteric artery: An additional dynamic sonographic feature of midgut volvulus. Pediatr Radiol. 1991;21:156-157.
- Sze RW, Guillerman RP, Krauter D, Evans AS. A possible new ancillary sign for diagnosing midgut volvulus: The truncated superior mesenteric artery. J Ultrasound Med.2002;21:477-480.
- Hiorns MP. Gastrointestinal tract imaging in children: Current techniques. Pediatr Radiol. 2011;41:42-54.
- Reed AA, Michael K. Hypertrophic pyloric stenosis. J Diag Med Sonography. 2010;26:157-160.
- Costa Dias S, Swinson S, Torrão H, et al. Hypertrophic pyloric stenosis: Tips and tricks for ultrasound diagnosis. Insights Imaging. 2012;3:247-250.
- Del-Pozo G, Albillos JC, Tejedor D, et al. Intussusception in children: Current concepts in diagnosis and enema reduction. Radiographics. 1999;19:299-319.
- Kairam N, Kaiafis C, Shih R. Diagnosis of pediatric intussusception by an emergency physician-performed bedside ultrasound: A case report. Pediatr Emerg Care. 2009;25:177-180.
- Bahadır Ülger FE, Ulger A, Karakaya AE, et al. An easy, safe and effective method for the treatment of intussusception: Ultrasound-guided hydrostatic reduction. Ulus Travma Acil Cerrahi Derg. 2014;20:127-31.doi:10.5505/tjtes.2014.37898. Turkish.
- Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
- Cassar S, Bhatt S, Paltiel HJ, Dogra VS. Role of spectral Doppler sonography in the evaluation of partial testicular torsion. J Ultrasound Med. 2008;27:1629-1638.
- Stengel JW Remer EM. Sonography of the scrotum: Case-based review. AJR Am J Roentgenol. 2008;190:S35-S41.
- Dogra VS, Gottlieb RH, Oka M, Rubens RJ. Sonography of the scrotum. Radiology. 2003;227:18-36.
- Frush DP, Sheldon CA. Diagnostic imaging for pediatric scrotal disorders. Radiographics. 1998;18:969-985.
- Baldisserotto M, de Souza JCK, Pertence AP, Dora MD. Color Doppler sonography of normal and torsed testicular appendages in children. AJR Am J Roentgenol. 2005;184:1287-1292.
- Sung EK, Setty BN, Castro-Aragon I. Sonography of the pediatric scrotum: Emphasis on the Ts-torsion, trauma, and tumors. AJR Am J Roentgenol. 2012;198: 996-1003.
- Carkaci S, Ozkan E, Lane D, Yang WT. Scrotal sonography revisited. J Clin Ultrasound. 2010;38:21-37.
- Bhatt S, Dogra VS. Role of US in testicular and scrotal trauma. Radiographics. 2008;28:1617-1629.
- Servaes S, Victoria T, Lovrenski J, Epelman M. Contemporary pediatric gynecologic imaging. Semin Ultrasound CT MRI. 2010;31:116-140.
- Stranzinger E, Strouse PJ. Ultrasound of the pediatric female pelvis. Semin Ultrasound CT MRI. 2008;29:98-113.
- Cohen HL, Bober SE, Bow SN. Imaging the pediatric pelvis: the normal and abnormal genital tract and simulators of its diseases. Urol Radiol. 1992;14:273-283.
- Garel L, Dubois J, Grignon A, et al. US of the pediatric female pelvis: A clinical perspective. Radiographics. 2001;21:1393-1407.
- Shadinger LL, Andreotti RF, Kurian RL. Preoperative sonographic and clinical characteristics as predictors of ovarian torsion. J Ultrasound Med. 2008;27:7-13.
- Nievelstein RAJ, Robben SGF, Blickman JG. Hepatobiliary and pancreatic imaging in children-techniques and an overview of non-neoplastic disease entities. Pediatr Radiol. 2011;41:55-75.
- Babcock DS. Sonography of the acute abdomen in the pediatric patient. J Ultrasound Med. 2002;21:887-899.
- Berrocal T, Prieto C, Pastor I, et al. Sonography of pancreatic disease in infants and children. Radiographics. 1995;15:301-313.
- Finnell SME, Carroll AE, Downs SM, Subcommittee on Urinary Tract Infection. Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128: e749-e770.
- Stogianni A, Nikolopoulos P, Oikonomou I, et al. Childhood acute pyelonephritis: comparison of power Doppler sonography and Tc-DMSA scintigraphy. Pediatr Radiol. 2007;37:685-690.
- Brader P, Riccabona M, Schwarz T, et al. Value of comprehensive renal ultrasound in children with acute urinary tract infection for assessment of renal involvement: comparison with DMSA scintigraphy and final diagnosis. Eur Radiol. 2008;18: 2981-2989.
- Avni FE, Garel C, Cassart M, et al. Imaging and classification of congenital cystic renal diseases. AJR Am J Roentgenol. 2012;198:1004-1013.
- Wootton-Gorges SL1, Thomas KB, Harned RK, et al. Giant cystic abdominal masses in children. Pediatr Radiol. 2005;35: 1277-1288.
- Hamza M, Bodensteiner JB, Noorani PA, Barnes PD. Benign extracerebral fluid collections: A cause of macrocrania in infancy. Pediatr Neurol. 1987;3:218-221.
- Zahl SM, Egge A, Helseth E, Wester K. Benign external hydrocephalus: A review, with emphasis on management. Neurosurg Rev. 2011;34: 417-432.
- Chen CY, Chou TY, Zimmerman RA, et al. Pericerebral fluid collection: Differentiation of enlarged subarachnoid spaces from subdural collections with color Doppler US. Radiology. 1996;201:389–392.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: A comprehensive review. J Pediatr Orthop B. 2014;23:32-36.
- Eich GF, Superti-Furga A, Umbricht FS, Willi UV. The painful hip: Evaluation of criteria for clinical decision-making. Eur J Pediatr. 1999;158:923-928.
- Zawin JK, Hoffer FA, Rand FF, Teele RL. Joint effusion in children with an irritable hip: US diagnosis and aspiration. Radiology. 1993;187:459-463.
- Liberman B, Herman A, Schindler A, et al. The value of hip aspiration in pediatric transient synovitis. J Pediatr Orthop. 2013;33:124-127.
- Dodwell ER. Osteomyelitis and septic arthritis in children: Current concepts. Curr Opin Pediatr. 2013;25:58-63.
- Thapa M, Vo JN, Shiels WE 2nd. Ultrasound-guided musculoskeletal procedures in children. Pediatr Radiol. 2013;43 Suppl 1:S55-60.
- Shiels WE 2nd. Soft tissue foreign bodies: Sonographic diagnosis and therapeutic management. Ultrasound Clinics. 2007;2: 669-681.
- Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin: Springer, 2007.