Use of contrast in CTA and CTV of the kidneys
Dr. Herts is Head of the Section of Abdominal Imaging, Division of Radiology, Cleveland Clinic Foundation, Cleveland, OH.
The clinical uses of computed tomographic angiography (CTA) and CT venography (CTV) in the urinary tract fall into three broad categories. The first is in the examination of renal arterial disease, including renal artery stenosis and stents, and renal aneurysms. The second is in the assessment of renal vascular anatomy, for surgical planning prior to nephron-sparing surgery, evaluation of living renal transplant donors, or examination of crossing vessels in ureteropelvic junction obstruction. The third is in the examination of renal vein thrombosis, including tumor thrombus.
In any CT examination of the kidney, certain considerations must be kept in mind. In many examinations, it is necessary to image both the renal arterial and renal venous systems. The examination necessarily includes the renal parenchyma; therefore, it is important to follow basic renal imaging principles, including the use of a consistent slice thickness and collimation for all scan phases and the incorporation of techniques that enable identification of calcifications and characterization of renal lesions. It is also important to determine whether the examination will include the collecting system. Furthermore, the kidneys may differ in size, position, and function. Renal vascular anomalies are common and highly variable.
The kidneys actively concentrate contrast. Poor kidney function is often associated with a reduction in blood supply, which may limit the effectiveness of CTA. Therefore, adequate renal function is often necessary for disease detection. Finally, contrast material must be used with care in patients with renal in-sufficiency to reduce the risk of contrast-associated nephropathy.
Contrast nephropathy
Various definitions have been used to describe contrast-associated nephropathy. It has been reported in the literature as an increase above the baseline creatinine level of 20% to 50%, or an absolute increase in serum creatinine of 0.5 to 2.0 mg/dL. 1,2
There are many risk factors for contrast-associated nephropathy, including pre-existing renal insufficiency (serum creatinine >1.5 mg/dL), diabetes mellitus, age >70 years, and dehydration. 1-7 The combination of diabetes and pre-existing renal insufficiency poses the highest risk for contrast-asso-ciated nephropathy, as much as 5 times the general risk. 3
In most cases, contrast nephropathy is self-limiting and its clinical course is predictable. Typically, the serum creatinine level begins to rise within 24 hours of contrast exposure, peaks within 96 hours (4 days), and then returns to baseline, with little residual effect. 1 Therefore, concern over the possibility of contrast-associated nephropathy should not preclude a contrast-enhanced CT examination in a patient who needs it.
At the Cleveland Clinic, our practice when performing CTA and CTV of the kidneys in patients with a normal serum creatinine level (<1.5 mg/dL) is to use 150 mL of low-osmolar, nonionic contrast media (Table 1). Given an iodine concentration of 300 mg/mL, this results in a total iodine load of 45 g.
In patients with a mildly elevated serum creatinine level (1.5 to 1.9 mg/dL) and no other risk factors, we use our standard low-osmolar nonionic contrast agent and standard contrast load, but hydrate with oral fiuids after the procedure to ensure that dehydration does not increase the risk of contrast-associated nephropathy. If the patient has additional risk factors, including diabetes, older age, or previous renal insufficiency, we begin hydration prior to the procedure and consider using an isosmolar contrast agent, such as iodixanol.
In patients with moderate renal insufficiency (serum creatinine 2.0 to 2.4 mg/dL), it is important to consider using magnetic resonance imaging (MRI) or ultrasound (US) as an alternative imaging method. If contrast-enhanced CT is necessary, the patient should undergo intravenous (IV) hydration before the procedure, and oral or IV hydration afterward. The patient should also receive an isosmolar contrast agent to minimize the risks of contrast-associated nephrotoxicity.
In patients with a serum creatinine level >2.5 mg/dL, contrast-enhanced CT is generally not recommended, as poor renal function will likely preclude adequate assessment of the kidneys and renal vasculature. MRI and US are acceptable alternatives in most patients. When CT is necessary, it is essential to hydrate the patient intravenously both before and after the procedure and use an isosmolar contrast agent.
Intravenous hydration may be contraindicated in patients with severe heart failure or other conditions that require fiuid restriction. In general, however, outpatients who require hydration receive a 3-hour infusion of 500 mL of half-normal saline before the examination and are instructed to push oral fiuids afterward.
It is possible to control hydration more closely in hospitalized patients. At our institution, inpatients receive an infusion of half-normal saline at 100 mL/hr for 12 hours before, and 12 hours after, the examination, 8,9 an approach that has been developed in association with our team of nephrologists.
Contrast injection protocols
Factors to consider in developing contrast injection protocols include the rate of injection, contrast viscosity, scan duration, injection duration, contrast volume, and cost. When CTA is performed alone, it is preferable for the injection duration and scan duration to be equivalent to maintain high levels of con
trast enhancement, regardless of the scan delay. If, however, CTA is performed in combination with diagnostic CT, it will be necessary to inject a certain minimum iodine load to enable imaging of the liver and kidneys. For studies that combine diagnostic CT with CTA, we use 150 mL of 300 mg I/mL contrast material. After a 20-mL test bolus, we inject the remaining 130 mL at 4 mL/sec for approximately 30 seconds (Table 2).
For renal CTA alone, we use a higher-concentration agent (370 mg I/mL) and reduce the intravenous injection rate to 3 to 3.5 mL/sec to accommodate the higher viscosity of the contrast material. Total contrast volume is approximately 100 mL, and injection duration is approximately 33 seconds.
With most studies, we determine optimal scan timing through injection of a test bolus of contrast. Scanning the upper abdominal aorta, we use a single slice and no table movement. Starting 10 seconds after contrast injection, we scan every second for 30 seconds. The time to peak enhancement determines the scan delay; however, 5 seconds should be added to the scan delay if the study will include examination of the renal veins.
Use of a 20-mL saline fiush can improve enhancement when injecting low volumes of high-concentration contrast media. We do not routinely use a saline fiush when CTA studies are performed with a 300 mg I/mL contrast agent. We do, however, routinely hand-inject a 100-mL saline fiush during CT urography, as we find that it results in better contrast opacification of the collecting system.
Protocols
The three-phase helical CT scan we routinely use to image the kidneys consists of an unenhanced phase, a vascular or corticomedullary phase (which is useful for performing both CTA and CTV), and a nephrographic or parenchymal phase (which can also be used for performing CTV).
The unenhanced CT scan localizes the kidneys in anticipation of the contrast-enhanced examination. Calcifications can also be seen during the unenhanced phase. These may be renal calculi or vascular calcifications, such as at the renal artery ostia or in an aneurysm. Calcifications may also be present in the wall or septae of a complex cyst. The unenhanced scan is also required for renal lesion characterization, as it provides the baseline attenuation for assessing en-hancement after contrast.
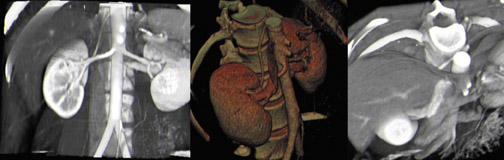
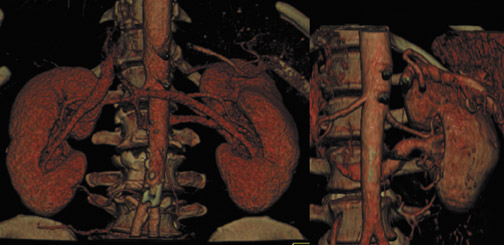
The vascular-phase scan generally extends from above the celiac axis through the common iliac arteries. 10 Recently, we have expanded the scan range to include the diaphragm, as renal veins and arteries are widely variable in both their origin and course (Figure 1). The renal arteries can arise from anywhere along the abdominal aorta or even the common iliac arteries.
During the corticomedullary or vascular phase, scanning is timed to coincide with renal arterial and venous enhancement. Generally, the scan delay is approximately 20 to 35 seconds after injection, but the exact timing is best determined with a timing bolus. In the corticomedullary or vascular phase, the renal cortex enhances, but there is little contrast concentration by the renal medulla. Contrast will be observed in the collecting system only if a preload or timing bolus is used.
The nephrographic or parenchymal phase is most sensitive for the detection and characterization of renal lesions. 11,12 It also provides more consistent opacification of the renal veins and the inferior vena cava (IVC). Therefore, this is often the best phase to use for imaging of the branch vessels, such as the adrenal, lumbar, and gonadal veins.
Scanners
In the past, we used a 4-detector-row scanner for renal CTA. Today, however, we scan renal patients on a 16-row multidetector CT scanner whenever possible. In addition to providing greater image detail, the 16-row scanner enables much more rapid reconstruction and avoids tube-cooling delays.
With the 16-row scanner, we use a 0.75-mm collimation for all 16 rows, which results in a table movement of 12 mm per rotation. At 0.5 seconds per revolution, and a coverage of 24 mm/sec, we can complete the vascular phase of the scan relatively quickly. Standard settings include 120 kV and an effective mA of 200.
After image acquisition, we create 2 sets of images. One, a diagnostic set intended for review on the picture archiving and communications systems (PACS) and for filming, uses 3-mm-thick slices and a 3-mm reconstruction interval. Creating this set of images takes no additional time on our scanner (Siemens Sensation 16, Siemens Medical Solutions, Malvern, PA). The second set of images, intended for both two- (2D) and three-dimensional (3D) CTA, uses 1-mm-thick slices and a 0.8-mm reconstruction interval.
With the 4-detector-row scanner, we use 2.5-mm collimation for all 4 rows and a table speed of 10 mm per rotation. At 0.5 seconds per rotation, the resulting 20 mm/sec coverage is only slightly less than that achieved with our 16-row scanner. It is also possible to use a 1-mm collimation and a table movement of 4 mm per rotation, which results in a coverage of 8 mm/sec at a 0.5-second rotation time. This approach results in one-third the coverage of a 16-row scanner, however, and prohibits scanning from the diaphragm through the iliac arteries.
With the 4-detector-row scanner, we create 1 set of diagnostic images for PACS review, filming, and 2D and 3D CTA, using 3-mm-thick slices and a 1.5-mm reconstruction interval.
Clinical indications
Renal arterial disease
The imaging of renal arterial disease encompasses renal artery stenosis, renal artery aneurysms, and renal vascular diseases, such as fibromuscular dysplasia. Renal artery stenosis remains a difficult imaging challenge. A thorough assessment requires both anatomic and functional information, which may not be possible with a single test. 13-16
A key goal of noninvasive imaging of renal artery stenosis is to avoid renal arteriography. Although arteriography is considered the "gold standard" for the assessment of renal artery stenosis, the risks of an invasive procedure may not be justified, given the low prevalence of renal artery stenosis as a cause of hypertension (3% to 5%). 14 At the same time, noninvasive assessment must achieve a high sensitivity to be an effective screening study.
According to the American College of Radiology criteria for the radiologic investigation of renal artery stenosis, this condition is defined as a reduction in vessel diameter of >50%. 13 Without functional information, however, the diagnosis is actually achieved in reverse: If treatment reduces the blood pressure, then the diagnosis of renal artery stenosis is considered, in retrospect, to have been correct.
There are several options for the diagnosis of renal artery stenosis, including US, MR angiography, CTA, digital subtraction angiography, and measurement of selective renal vein renins. 17 Ultrasound is noninvasive, inexpensive, and does not require iodinated contrast media or radiation. Its sensitivity, however, is reported in the scientific literature to range from 0% to 90% based on peak systolic velocities and the parvus-tardus waveform. 18-20 Gadolinium-enhanced MRA is highly sensitive in the proximal renal vessels (77% to 100%) 21,22 and is useful in patients with reduced renal function.
CT angiography requires the use of radiation and iodinated contrast media. It is sensitive in the proximal vessel as well, with a sensitivity for stenosis of 88% to 96%. 23-25 Its overall use is limited, however, because many patients with suspected renal artery stenosis have renal insufficiency and are at risk for contrast-associated nephrotoxicity.
It is important to note that the evaluation of renal artery stenosis is complicated by the existence of multiple renal arteries in 25% to 33% of patients, or 15% to 25% of kidneys. Such anatomical features as a small accessory artery, an early branch artery, or 2 closely situated branch arteries present major imaging challenges, particularly for US.
The CT evaluation of renal artery stent patency calls for the use of either coronal multiplanar reformations (MPRs) or curved MPRs to depict the entire renal artery. CT angiography can depict the interior of the stent as well.
CT angiography can easily detect renal artery aneurysms. In addition, by depicting the neck of the aneurysm and branch vessels, it can help target surgical treatment or embolization. It is important that the examination include 3D renderings, however. A bright area of contrast on the axial images may be mistaken for the renal pelvis, for example, whereas on 3D imaging the same finding is more easily indentified as an aneurysm in the renal hilum (Figure 2).
We generally reconstruct CTA data as thin-slice maximum intensity projections (thin MIPs) or MPRs, as it eases visualization of curved structures that run in and out of a scan plane. It is possible to scroll back and forth through a series of thin-slab MIPs, easily identifying the renal vasculature. This approach also eliminates the need for editing when doing MPRs.
Surgical planning
The Cleveland Clinic Foundation is a major referral center for nephron-sparing surgery. The evaluation of candidates for this procedure is the most common reason for performing renal CTA and CTV in our institution. 26,27 Nephron-sparing surgery is indicated in patients with renal neoplasms who would otherwise require dialysis after conventional surgery, or radical nephrectomy. Such patients may have a solitary functional kidney, bilateral renal tumors, or underlying renal disease that would necessitate dialysis following radical nephrectomy.
The purpose of CTA and CTV in such cases is to localize all of the arteries, veins, and major branch vessels. It is important to remember that the renal arteries are end-arteries; therefore, preserving renal function necessitates preserving the renal arterial supply.
There are 4 segmental branches of the renal artery, each named for the renal parenchymal segment it supplies. These branches are the apical, basilar, anterior, and posterior segmental branches. With renal CTA, it is usually possible to identify at least 3-and often all 4-of these segmental renal branch vessels.
Renal arterial anatomy is highly variable. Accessory right renal arteries can course anterior to the IVC, rather than in the normal posterior position. A patient may have multiple renal arteries on one or both sides (Figure 3). Even in the case of a single renal artery, the point of origin can vary widely from patient to patient, so it is important that the scan extend through the common iliac arteries.
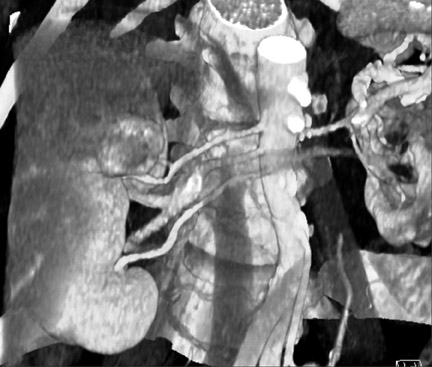
It is also important to look for early branch vessels. Some branches, such as the apical polar branch shown in Figure 4, go directly into the renal cortex, rather than entering at the renal hilum. Others may originate near the renal artery ostia, an anatomical variation that the surgeon must be aware of when clamping vessels.
Renal vein anatomy
The right renal vein may be a single vessel or multiple vessels, and may enter the IVC from anterior, lateral, or posterior positions. The right gonadal vein typically enters the IVC directly, but in some cases joins an inferior accessory right renal vein.
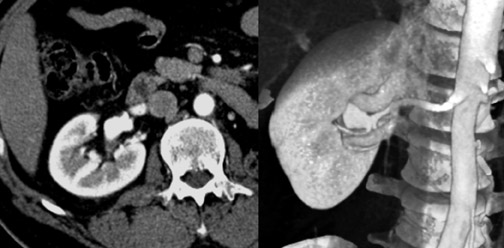
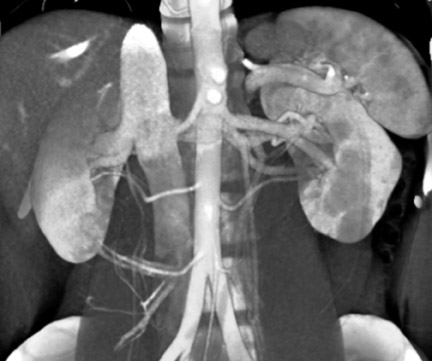
The left renal vein usually courses anterior to the aorta, with the adrenal and gonadal veins emptying directly into it. There are, however, circumaortic and retroaortic variants of the left renal vein (Figure 5), as well as variants of the adrenal and gonadal vein anatomy.
Living renal donors
Multiple studies have shown that CTA and MRA compare favorably with angiography for the evaluation of potential living renal transplant donors. 28,29 CTA yields better venous assessment and, when combined with 3D reconstructions, better depicts anatomic relationships. 30 In addition, CTA is a single, minimally invasive test that can replace a combination of tests, such as US or conventional CT combined with angiography and postangiography intravenous urography. Thus, the use of CTA reduces costs and inconvenience to the donor.
When evaluating a potential renal transplant donor, the primary goal is to ensure the health of the donor, thus each donor first completes a full medical screening. Once they are determined to be a suitable donor candidate, each donor then undergoes an assessment of the renal vascular anatomy to determine the preferred side for donation in order to maximize the likelihood of graft success.
In potential donors with a single artery and conventional venous anatomy, the left side is preferred because of the longer left renal vein. The disadvantage of using the left kidney is the presence of the adrenal, gonadal, and lumbar renal vein branches. If one of the donor's kidneys has a cyst or a small calculus, that kidney will be the one selected for transplantation.
As for renal vein anomalies, it is not crucial to preserve small branch vessels in renal donation, as collateral venous fiow will be sufficient. The short right renal vein remains a problem, however, prompting surgeons to avoid selecting the right kidney for donation.
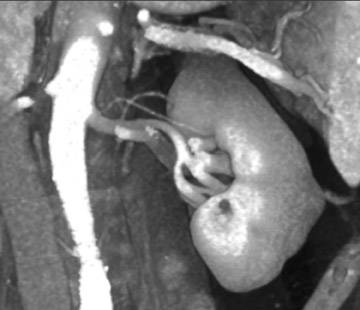
The approach to multiple renal arteries (Figure 6) will depend on their proximity to the renal ostia. An early arterial branch (within 1 to 2 cm of the renal ostia) will usually be treated as an accessory artery. An accessory artery may be anastomosed to the main renal artery or graft. Small segmental accessory arteries are likely to be sacrificed, however.
UPJ obstruction
Renal CTA and CTV are important tools in the evaluation of ureteropelvic junction (UPJ) obstruction and are used in the selection of the most appropriate treatment method. Endourologic repair has a lower success rate in patients with crossing vessels at the UPJ. 31 Intravenous urography, angiography, or endoluminal US can be used to evaluate UPJ obstruction, but CTA is the preferred examination, as it is minimally invasive and provides direct visualization of both the UPJ and vessels. The size and location of vessels are important in the evaluation of UPJ vasculature. Of interest are branch or accessory arteries or veins with a diameter of >2 mm, and any vessel located within 1 to 2 cm of the UPJ. 32,33
Renal CTV
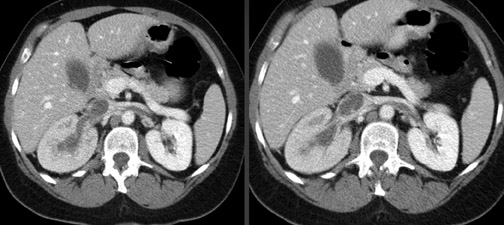
CT venography for the evaluation of renal vein thrombosis is often requested in conjunction with a conventional CT of the abdomen and pelvis. Aside from renal cell carcinoma, which can cause renal vein thrombus, the most common causes for renal vein thrombosis are nephrotic syndrome, malignancy, and hypoalbuminemia (Figure 7).
Postprocessing
For renal CTA and CTV, we usually use MIP reconstructions of coronal oblique slices angled parallel to the aorta or IVC. The slice thickness is 3 mm, and the reconstruction interval is 2.5 mm. Thin-slab MIPs avoid the need for editing and enable a technologist, using a predefined protocol, to create a series of images to scroll through.
Volume rendering is the most sophisticated reconstruction technique and the hardest to learn. It is also the most fiexible rendering technique and can provide quite specific anatomic detail. It is possible to vary the windows and levels to look at either the arterial system or the venous system.
Occasionally, we use MPRs or curved MPRs of CTA and CTV data. As with thin-slab MIPs, this technique produces a series of images for review. It is more difficult to draw curved planes accurately, however.
Generally, technologists do most of the MIPs and MPRs at our institution, whereas physicians do the volume renderings, as well as some MPRs and MIPs. We prefer to save CT examinations with a large number of images to a CD-ROM, which we give to referring physicians. This enables us to give referring physicians the entire set of images, including a full set of MIPs.
When preparing laser film instead of CDs for referring physicians, we suggest printing only selected images. Digital image files and movies are alternatives to film. These, however, require electronic transfer, which is more technology-intensive.
Optimizing CTA
One of the first steps in optimizing CTA is to get the technologist involved. The use of preset protocols that specify collimation, slice thickness, and reconstruction interval enables the technologist to acquire the same high-quality data set every time. It is also possible to use preset reconstruction protocols, but it is essential to educate technologists as to what the CTA should look like in the end.
It is also important to work with the application specialist to learn how to get the optimal performance from the CT scanner and workstations. Frequent practice at the workstation is also critical.
Finally, talk with referring physicians. Finding out what their specific needs and interests are-and fulfilling those needs-is essential to increasing CTA referrals.
Discussion
ELLIOT K. FISHMAN, MD: That was terrific, Brian. Are there any questions from the panel?
LEO P. LAWLER, MD, FRCR: Thank you, Brian; that was very comprehensive. But I have two questions. You probably have the same experience as we do, in that CTA is often performed with something else. They want to see the renal parenchyma as well as the vasculature, etc. I had a question about partial nephrectomy patients with follow-up. Do you think the examinations require noncontrast, arterial, and venous enhancement images of the renal parenchyma to look for recurrence, as well as just the simple single-phase image?
BRIAN R. HERTS, MD: It depends upon how soon after the surgery you are talking about. My experience with our surgeon has been that recurrences, especially with the small tumors, do not really occur until 2 or 4 years after surgery. So, if the follow-up is being done in the first 6 months, you can just do a standard abdomen/pelvis CT.
LAWLER: I also have a second question, also about a group of patients in whom clinicians want both the CTA and the follow-through CT urogram, such as in living donors. Despite the fact that CT does not have the line-pair resolution of an intravenous urogram, do you think that performing 3D processing after the arterial-venous phase may obviate the need for direct visualizations of the urothelium in a patient with hematuria?
HERTS: Our CT urogram protocol is much more delayed than our CTA and CTV. Our urogram protocol takes scans out to 8 and 10 minutes with or without compression, and we use a saline chaser, so we get a much better look at the urothelium. We would add that to this protocol if that were the indication.
GEOFFREY D. RUBIN, MD: Brian, your discussion of the treatment of patients with various degrees of azotemia is very useful. Have you considered the use of renal protective agents, particularly acetylcysteine and fenold-opam, in your protocol? There has been a lot of controversy in the literature about the relative benefits.
HERTS: We came up with a hospital-wide policy, in conjunction with the nephrologists and the vascular surgeons. We do consider the use of acetylcysteine, although it is not recommended or mandatory. We had theophylline on our list 5 years ago, and we have now removed it as the controversies arose. So, we put that as a guideline, but we do not mandate it now.
RUBIN: Since formalizing your protocol, have you followed patients to note the frequency of contrast-induced nephrotoxicity, particularly in the groups of the higher azotemics that you delineated?
HERTS: We have not formally followed them. We talk to urologists a fair amount because so many of these patients had elevated creatinines and are going on to partial nephrectomy. But that is a redundant reason: that is why they have elevated creatinines and that is why they need partial nephrectomies. It is obviously complicated by surgery, but the patients are actually doing quite well and generally do not have a problem. Most of the experience with contrast-induced nephropathy when you are talking to nephrologists comes out of the cardiac catheterization laboratory; very little comes from the radiology department.
RUBIN: Do you think we might be overly conservative in this regard?
HERTS: I think we are.
W. DENNIS FOLEY, MD: Brian, I have a quick question for you about preparing patients with diabetes who are taking metformin. Is your protocol to take them off metformin for 48 hours after the procedure, as most people do? Then do you remeasure their creatinine?
HERTS: If they have a normal creatinine, we do exactly that. We have them stop taking metformin; they can take it up to the day of the procedure. Then, we stop it for 48 hours; we do not routinely re-measure creatinine.
U. JOSEPH SCHOEPF, MD: You mentioned the use of saline chasing in your protocols. Can you quickly explain what exactly its role is in the imaging of the renal vasculature in your institution? What systems do you use it for?
HERTS: Most of the time, we are really trying to image the renal parenchyma at the same time. So, in those cases, we give a full load of contrast and the saline chaser is not as important. If we are really just looking at the renal vasculature and we have a smaller volume of contrast, then we will use a saline chaser, just 20 mL, because we are using a much smaller volume of contrast. That has been shown to maintain the contrast level in the blood vessels by clearing the line and getting a little bolus behind it.
SCHOEPF: What is the role of the saline injection? You mentioned an in-jection for a CT urography.
HERTS: It actually provides a little bit of a diuresis, and we are still collecting our data. We initially started with 50 mL and thought it was simple, that they would inject from a 50-mL syringe. But we were not getting the opacification that we wanted. When we increased the saline injection volume to 100 mL, we were actually getting much better contrast opacification in the collecting system, much more consistently.
FISHMAN: You mentioned that you use 300 mg I/mL concentration contrast routinely, except when you are doing dedicated vessel studies. Can you ex-plain your rationale for that?
HERTS: Part of the issue for us is that we are also looking at the liver and other organs as well. Our volume of contrast injection has been 150 mL. If we go down to the smaller doses, we tend to get a little less iodine in if we go to a higher concentration, unless we increase the volume, which we did not really want to do. That, obviously, would drive up costs. The other issue for us is that when you use the higher concentration agents, the contrast tends to get a little brighter in the kidneys, and I think you get a little more image artifact off the renal cortex.
FISHMAN: We typically use 350 mgI/mL contrast. We do not really change it based on the specific application. My experience has been similar to yours at the higher contrast concentration. The point is that it is actually a disadvantage to have higher concentration than 350 mgI/mL for two reasons: 1) the hyperviscosity, which has the problems with injecting at higher rates, and 2) artifact off the images.
HERTS: You really have to work to manipulate the windows and levels to see a small lesion in the renal cortex if you are using a high-concentra-tion contrast agent. You can not use the automatic soft-tissue windows that you pull up the images with; so you have to look very carefully for renal lesions in those patients.
FISHMAN: Some people have suggested that we could use even higher concentration contrast. Do you think that would have any value, or would it just be more of a detriment?
HERTS: If you are talking specifically about vasculature, the higher the concentration and the higher the density, the better the rendering will come out. But we are not just talking about the vasculature or just talking about the kidneys, so I do find it a detriment.
We do use the higher concentration agents for just the vascular studies and we will do that when we are doing an endovascular stent and we will push ourselves to use the 370 mgI/mL. But, we are trying to keep it simple for our technologists; so if we are doing a routine study, we use 300 mgI/mL contrast for the kidneys. All of our kidney studies are actually done as three-phase imaging now; we do not differentiate between different indications for renal imaging.
SCHOEPF: What recommendation can you offer as to what HU attenutation you want to achieve, especially for renal vasculature imaging? In cardiac imaging, there is a level of HU attenuation that you want to achieve to evaluate the vessel but not obscure any high attenuation lesion. Is that similar in renal vasculature?
HERTS: That is an interesting question. I have not looked at the actual attenuation value. I think we get lucky in the kidneys because the vein is always a little less dense than the artery because of the filtering in the kidneys, so it actually helps the differentiation there and with the volume-rendering techniques. I would estimate that once you get above 250 HU, it is never an issue.
FOLEY: I would like to raise a point relating to higher concentration contrast and the degree of attenuation. The issue is the kVp that we are using, particularly with the thin-section technique. As we get thin-section technique, we also are combining this with X-ray tubes that now have the capacity to deliver 700 mAs to compensate for the very fast scan rotation speeds we are using. Do you have any advice, Brian, in terms of the kVp, that you think would be optimal for CTA, in conjunction with the concentration of contrast you would use?
HERTS: I actually have not spent any time experimenting, but I know that our scanners do 100 kVp--it is supposedly very high attenuation and it really does change what you come up with. The problem I have found with characterizing renal lesions is that I do not know what is going to happen. I have not gotten a firm answer from the scanner manufacturer as to what will happen when you look at a density value on a renal lesion at 100 kVp. That is really been my big concern because that is one of our biggest uses. That is certainly something that needs to be considered, but I do not have an answer for you.
RUBIN: I could speak to that briefiy because we recently did a little study of that. We took some tubes of contrast and we changed the kVp while keeping the computed tomography dose index constant. We found that, although the contrast solution gets brighter when you lower the kVp and you get more of a difference between soft tissue and an enhanced vessel, for example, the noise goes up out of proportion. So, in fact, the contrast-to-noise ratio drops, even though the contrast goes up. So, if we are thinking of keeping the same radiation exposure, going to lower kVp, although increasing the absolute contrast, hurts us by increasing the noise to a greater extent. To really take advantage of the greater contrast difference of low kVp, we have to give the patients higher radiation exposure.
FISHMAN: That is interesting. Maybe we will come back to it later. There have been several suggestions that by dropping the kVp to 100 and raising the mAs appropriately, you would actually give 30% less radiation dose to achieve better or the same image quality. I think there are a couple of papers coming out on that. So I think you are right, that will be an area of a lot of interest, because it is potentially a way to do better CTA at a lower dose. Maybe your experience is different, but I have seen some data presented that suggest substantial decreases in radiation dose.
Have you done any work using gadolinium for renal imaging in patients with poor renal function?
HERTS: We have used it on occasion, but not as a routine. We have used it most of the time for stent studies for endovascular stents--not for the kidneys--because we can do similar evaluations for MR for nephron-sparing surgery with CTA.
